Allosteric loss-of-function mutations in HIV-1 Nef from a long-term non-progressor
- PMID: 17920628
- PMCID: PMC2117379
- DOI: 10.1016/j.jmb.2007.09.009
Allosteric loss-of-function mutations in HIV-1 Nef from a long-term non-progressor
Abstract
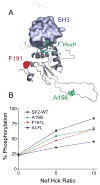
Activation of Src family kinases by human immunodeficiency virus type 1 (HIV-1) Nef may play an important role in the pathogenesis of HIV/AIDS. Here we investigated whether diverse Nef sequences universally activate Hck, a Src family member expressed in macrophages and other HIV-1 target cells. In general, we observed that Hck activation is a highly conserved Nef function. However, we identified an unusual Nef variant from an HIV-positive individual that did not develop AIDS which failed to activate Hck despite the presence of conserved residues linked to Hck SH3 domain binding and kinase activation. Amino acid sequence alignment with active Nef proteins revealed differences in regions not previously implicated in Hck activation, including a large internal flexible loop absent from available Nef structures. Substitution of these residues in active Nef compromised Hck activation without affecting SH3 domain binding. These findings show that residues at a distance from the SH3 domain binding site influence Nef interactions allosterically with a key effector protein linked to AIDS progression.
Figures



Similar articles
-
Interaction with the Src homology (SH3-SH2) region of the Src-family kinase Hck structures the HIV-1 Nef dimer for kinase activation and effector recruitment.J Biol Chem. 2014 Oct 10;289(41):28539-53. doi: 10.1074/jbc.M114.600031. Epub 2014 Aug 13. J Biol Chem. 2014. PMID: 25122770 Free PMC article.
-
Conserved residues in the HIV-1 Nef hydrophobic pocket are essential for recruitment and activation of the Hck tyrosine kinase.J Mol Biol. 2004 Nov 5;343(5):1255-68. doi: 10.1016/j.jmb.2004.09.015. J Mol Biol. 2004. PMID: 15491611
-
Oligomerization is required for HIV-1 Nef-induced activation of the Src family protein-tyrosine kinase, Hck.Biochemistry. 2004 Dec 21;43(50):15775-84. doi: 10.1021/bi048712f. Biochemistry. 2004. PMID: 15595833
-
Structure, function, and inhibitor targeting of HIV-1 Nef-effector kinase complexes.J Biol Chem. 2020 Oct 30;295(44):15158-15171. doi: 10.1074/jbc.REV120.012317. Epub 2020 Aug 29. J Biol Chem. 2020. PMID: 32862141 Free PMC article. Review.
-
Hck inhibitors as potential therapeutic agents in cancer and HIV infection.Curr Med Chem. 2015;22(13):1540-64. doi: 10.2174/0929867322666150209152057. Curr Med Chem. 2015. PMID: 25666803 Review.
Cited by
-
Hydrogen exchange mass spectrometry: what is it and what can it tell us?Anal Bioanal Chem. 2010 Jun;397(3):967-72. doi: 10.1007/s00216-010-3556-4. Epub 2010 Mar 1. Anal Bioanal Chem. 2010. PMID: 20195578 Free PMC article. Review.
-
Nef alleles from all major HIV-1 clades activate Src-family kinases and enhance HIV-1 replication in an inhibitor-sensitive manner.PLoS One. 2012;7(2):e32561. doi: 10.1371/journal.pone.0032561. Epub 2012 Feb 29. PLoS One. 2012. PMID: 22393415 Free PMC article.
-
Mass spectrometry coupled experiments and protein structure modeling methods.Int J Mol Sci. 2013 Oct 15;14(10):20635-57. doi: 10.3390/ijms141020635. Int J Mol Sci. 2013. PMID: 24132151 Free PMC article. Review.
-
Chemical library screens targeting an HIV-1 accessory factor/host cell kinase complex identify novel antiretroviral compounds.ACS Chem Biol. 2009 Nov 20;4(11):939-47. doi: 10.1021/cb900195c. ACS Chem Biol. 2009. PMID: 19807124 Free PMC article.
-
Immunogenicity of a lentiviral-based DNA vaccine driven by the 5'LTR of the naturally attenuated caprine arthritis encephalitis virus (CAEV) in mice and macaques.Vaccine. 2012 Apr 19;30(19):2956-62. doi: 10.1016/j.vaccine.2012.02.050. Epub 2012 Mar 2. Vaccine. 2012. PMID: 22387218 Free PMC article.
References
-
- Fackler OT, Baur AS. Live and let die: Nef functions beyond HIV replication. Immunity. 2002;16:493–497. - PubMed
-
- Greenway AL, Holloway G, McPhee DA. HIV-1 Nef: a critical factor in viral-induced pathogenesis. Adv Pharmacol. 2000;48:299–343. - PubMed
-
- Hanna Z, Priceputu E, Kay DG, Poudrier J, Chrobak P, Jolicoeur P. In vivo mutational analysis of the N-terminal region of HIV-1 Nef reveals critical motifs for the development of an AIDS-like disease in CD4C/HIV transgenic mice. Virology. 2004;327:273–286. - PubMed
-
- Joseph AM, Kumar M, Mitra D. Nef: “necessary and enforcing factor” in HIV infection. Curr HIV Res. 2005;3:87–94. - PubMed
-
- Kestler HW, III, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

