Iron homeostasis and toxicity in retinal degeneration
- PMID: 17921041
- PMCID: PMC2093950
- DOI: 10.1016/j.preteyeres.2007.07.004
Iron homeostasis and toxicity in retinal degeneration
Abstract
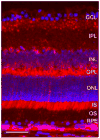
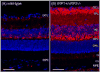
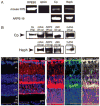
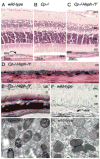
Iron is essential for many metabolic processes but can also cause damage. As a potent generator of hydroxyl radical, the most reactive of the free radicals, iron can cause considerable oxidative stress. Since iron is absorbed through diet but not excreted except through menstruation, total body iron levels buildup with age. Macular iron levels increase with age, in both men and women. This iron has the potential to contribute to retinal degeneration. Here we present an overview of the evidence suggesting that iron may contribute to retinal degenerations. Intraocular iron foreign bodies cause retinal degeneration. Retinal iron buildup resulting from hereditary iron homeostasis disorders aceruloplasminemia, Friedreich's ataxia, and panthothenate kinase-associated neurodegeneration cause retinal degeneration. Mice with targeted mutation of the iron exporter ceruloplasmin have age-dependent retinal iron overload and a resulting retinal degeneration with features of age-related macular degeneration (AMD). Post mortem retinas from patients with AMD have more iron and the iron carrier transferrin than age-matched controls. Over the past 10 years much has been learned about the intricate network of proteins involved in iron handling. Many of these, including transferrin, transferrin receptor, divalent metal transporter-1, ferritin, ferroportin, ceruloplasmin, hephaestin, iron-regulatory protein, and histocompatibility leukocyte antigen class I-like protein involved in iron homeostasis (HFE) have been found in the retina. Some of these proteins have been found in the cornea and lens as well. Levels of the iron carrier transferrin are high in the aqueous and vitreous humors. The functions of these proteins in other tissues, combined with studies on cultured ocular tissues, genetically engineered mice, and eye exams on patients with hereditary iron diseases provide clues regarding their ocular functions. Iron may play a role in a broad range of ocular diseases, including glaucoma, cataract, AMD, and conditions causing intraocular hemorrhage. While iron deficiency must be prevented, the therapeutic potential of limiting iron-induced ocular oxidative damage is high. Systemic, local, or topical iron chelation with an expanding repertoire of drugs has clinical potential.
Figures










References
-
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. - PubMed
-
- Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33:940–959. - PubMed
-
- Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet. 2002;360:516–520. - PubMed
-
- Aouad F, Florence A, Zhang Y, Collins F, Henry C, Ward RJ, Crichton R. Evaluation of new iron chelators and their therapeutic potential. Inorg Chim Acta. 2002;339:470–480.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

