Hindered diffusion through an aqueous pore describes invariant dye selectivity of Cx43 junctions
- PMID: 17921206
- PMCID: PMC2186237
- DOI: 10.1529/biophysj.107.115634
Hindered diffusion through an aqueous pore describes invariant dye selectivity of Cx43 junctions
Abstract
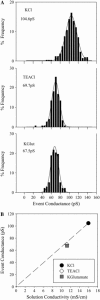
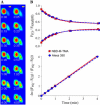
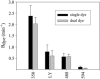
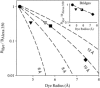
The permselectivity (permeance/conductance) of Cx43-comprised gap junctions is a variable parameter of junctional function. To ascertain whether this variability in junctional permselectivity is explained by heterogeneous charge or size selectivity of the comprising channels, the permeance of individual Cx43 gap junctions to combinations of two dyes differing in either size or charge was determined in four cell types: Rin43, NRKe, HeLa43, and cardiac myocytes. The results show that Cx43 junctions are size- but not charge-selective and that both selectivities are constant parameters of junctional function. The consistency of dye selectivities indicates that the large continuum of measured junctional permselectivities cannot be ascribed to an equivalent continuum of individual channel selectivities. Further, the relative dye permeance sequence of NBD-M-TMA approximately Alexa 350 > Lucifer yellow > Alexa 488 >> Alexa 594 (Stokes radii of 4.3 A, 4.4 A, 4.9 A, 5.8 A, and 7.4 A, respectively) and the conductance sequence of KCl > TEACl approximately Kglutamate are well described by hindered diffusion through an aqueous pore with radius approximately 10 A and length 160 A. The permselectivity and dye selectivity data suggest the variable presence in Cx43-comprised junctions of conductive channels that are either dye-impermeable or dye-permeable.
Figures




Similar articles
-
Regulation of gap junctional charge selectivity in cells coexpressing connexin 40 and connexin 43.Am J Physiol Heart Circ Physiol. 2009 Jul;297(1):H450-9. doi: 10.1152/ajpheart.00287.2009. Epub 2009 May 22. Am J Physiol Heart Circ Physiol. 2009. PMID: 19465552 Free PMC article.
-
The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities.Biophys J. 2004 Aug;87(2):958-73. doi: 10.1529/biophysj.103.036350. Biophys J. 2004. PMID: 15298902 Free PMC article.
-
Quantification of gap junction selectivity.Am J Physiol Cell Physiol. 2005 Dec;289(6):C1535-46. doi: 10.1152/ajpcell.00182.2005. Epub 2005 Aug 10. Am J Physiol Cell Physiol. 2005. PMID: 16093281
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Is the junctional uncoupling elicited in rat ventricular myocytes by some dephosphorylation treatments due to changes in the phosphorylation status of Cx43?Eur Biophys J. 2004 May;33(3):201-10. doi: 10.1007/s00249-003-0381-0. Epub 2004 Jan 27. Eur Biophys J. 2004. PMID: 14745523 Review.
Cited by
-
Quantifying phase separation at the nanoscale by dual-color fluorescence cross-correlation spectroscopy (dcFCCS).Biophys Rep. 2022 Feb 28;8(1):29-41. doi: 10.52601/bpr.2022.210026. Biophys Rep. 2022. PMID: 37287688 Free PMC article.
-
Fluid-phase and membrane markers reveal spatio-temporal dynamics of membrane traffic and repair in the green alga Chara australis.Protoplasma. 2021 Jul;258(4):711-728. doi: 10.1007/s00709-021-01627-z. Epub 2021 Mar 11. Protoplasma. 2021. PMID: 33704568 Free PMC article.
-
Support of Nerve Conduction by Respiring Myelin Sheath: Role of Connexons.Mol Neurobiol. 2016 May;53(4):2468-79. doi: 10.1007/s12035-015-9216-0. Epub 2015 Jun 2. Mol Neurobiol. 2016. PMID: 26033217
-
GABAergic responses of mammalian ependymal cells in the central canal neurogenic niche of the postnatal spinal cord.Neurosci Lett. 2013 Oct 11;553:57-62. doi: 10.1016/j.neulet.2013.07.007. Epub 2013 Jul 16. Neurosci Lett. 2013. PMID: 23872091 Free PMC article.
-
Mechanisms of phase-separation-mediated cGAS activation revealed by dcFCCS.PNAS Nexus. 2022 Jul 8;1(3):pgac109. doi: 10.1093/pnasnexus/pgac109. eCollection 2022 Jul. PNAS Nexus. 2022. PMID: 36741445 Free PMC article.
References
-
- Sohl G., Willecke K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004;62:228–232. - PubMed
-
- Evans W.H., Martin P.E. Gap junctions: structure and function. Mol. Membr. Biol. 2002;19:121–136. - PubMed
-
- Delorme B., Dahl E., Jarry-Guichard T., Briand J.P., Willecke K., Gros D., Theveniau-Ruissy M. Expression pattern of connexin gene products at the early developmental stages of the mouse cardiovascular system. Circ. Res. 1997;81:423–437. - PubMed
-
- Haefliger J.A., Castillo E., Waeber G., Bergonzelli G.E., Aubert J.F., Sutter E., Nicod P., Waeber B., Meda P. Hypertension increases connexin43 in a tissue-specific manner. Circulation. 1997;95:1007–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

