Diffusion of glycerol through Escherichia coli aquaglyceroporin GlpF
- PMID: 17921212
- PMCID: PMC2186255
- DOI: 10.1529/biophysj.107.115105
Diffusion of glycerol through Escherichia coli aquaglyceroporin GlpF
Abstract
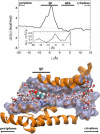
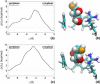
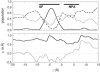
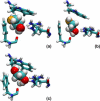
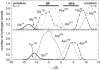
The glycerol uptake facilitator, GlpF, a major intrinsic protein found in Escherichia coli, selectively conducts water and glycerol across the inner membrane. The free energy landscape characterizing the assisted transport of glycerol by this homotetrameric aquaglyceroporin has been explored by means of equilibrium molecular dynamics over a timescale spanning 0.12 micros. To overcome the free energy barriers of the conduction pathway, an adaptive biasing force is applied to the glycerol molecule confined in each of the four channels. The results illuminate the critical role played by intramolecular relaxation on the diffusion properties of the permeant. These free energy calculations reveal that glycerol tumbles and isomerizes on a timescale comparable to that spanned by its adaptive-biasing-force-assisted conduction in GlpF. As a result, reorientation and conformational equilibrium of glycerol in GlpF constitute a bottleneck in the molecular simulations of the permeation event. A profile characterizing the position-dependent diffusion of the permeant has been determined, allowing reaction rate theory to be applied for investigating conduction kinetics based on the measured free energy landscape.
Figures

References
-
- Stroud R.M., Nollert P., Miercke L. The glycerol facilitator GlpF, its aquaporin family of channels, and their selectivity. Adv. Protein Chem. 2003;63:291–316. - PubMed
-
- Stroud R.M., Miercke L.J.W., O’Connell J., Khademi S., Lee J.K., Remis J., Harries W., Robles Y., Akhavan D. Glycerol facilitator GlpF and the associated aquaporin family of channels. Curr. Opin. Struct. Biol. 2003;13:424–431. - PubMed
-
- Agre P., Bonhivers M., Borgnia M.J. The aquaporins, blueprints for cellular plumbing systems. J. Biol. Chem. 1998;273:14659–14662. - PubMed
-
- Borgnia M., Nielsen S., Engel A., Agre P. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. 1999;68:425–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

