Recoil and stiffening by adherent leukocytes in response to fluid shear
- PMID: 17921217
- PMCID: PMC2186258
- DOI: 10.1529/biophysj.107.107102
Recoil and stiffening by adherent leukocytes in response to fluid shear
Abstract
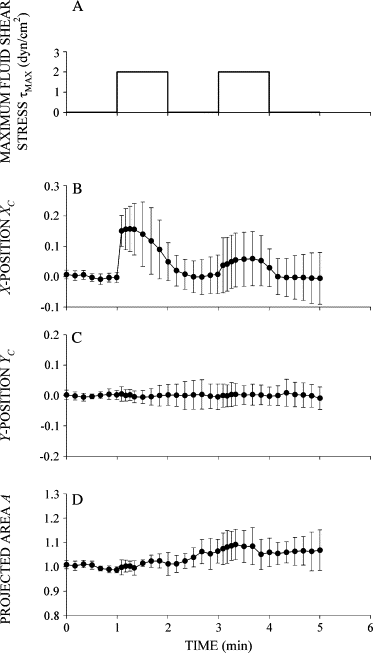
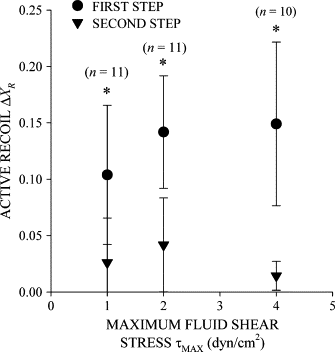
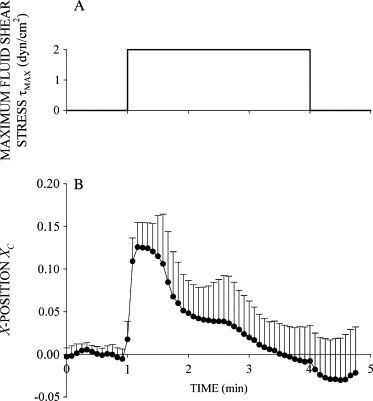
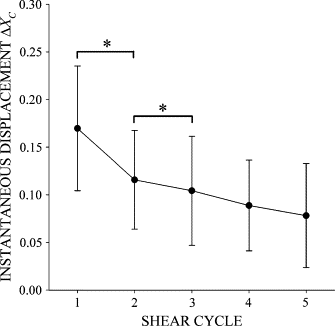
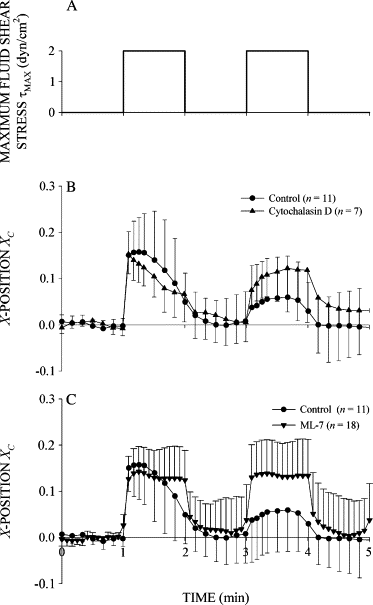
Prolonged exposure to fluid shear stress alters leukocyte functions associated with the immune response. We examined the initial response of freshly isolated human leukocytes to fluid shear stress under high magnification. Adherent leukocytes exhibit a rapid biomechanical response to physiological levels of fluid shear stress. After passive displacement in the direction of a constant fluid shear stress, adherent leukocytes actively recoil back in the opposite direction of the fluid flow. Recoil is observed within seconds of the applied fluid shear stress. Simultaneously, fluid shear stress induces a stiffening of the cell. The immediate cell displacement in response to a step increase in fluid shear stress is greatly attenuated in subsequent steps compared to the initial fluid shear stress step. Recoil is not mediated by actin polymerization-dependent mechanisms, as cytochalasin D had no effect on this early response. However, stiffening was determined in part by an intact actin cytoskeleton. Inhibiting myosin force generation with ML-7 abolished the recoil and stiffening responses, implicating force generation by myosin as an important contributor to the early leukocyte response to fluid shear stress. This initial shear stress response may be particularly important in facilitating leukocyte attachment under sustained fluid shear stress by the flowing blood in the microcirculation.
Figures

Similar articles
-
L-selectin shear thresholding modulates leukocyte secondary capture.Ann Biomed Eng. 2008 Apr;36(4):622-31. doi: 10.1007/s10439-008-9468-1. Epub 2008 Feb 26. Ann Biomed Eng. 2008. PMID: 18299990 Free PMC article.
-
The fluid shear stress distribution on the membrane of leukocytes in the microcirculation.J Biomech Eng. 2003 Oct;125(5):628-38. doi: 10.1115/1.1611515. J Biomech Eng. 2003. PMID: 14618922
-
Mechanisms for flow-enhanced cell adhesion.Ann Biomed Eng. 2008 Apr;36(4):604-21. doi: 10.1007/s10439-008-9464-5. Epub 2008 Feb 26. Ann Biomed Eng. 2008. PMID: 18299992 Free PMC article. Review.
-
Leukocyte fluid shear response in the presence of glucocorticoid.J Leukoc Biol. 2004 Apr;75(4):664-70. doi: 10.1189/jlb.1003464. Epub 2004 Jan 14. J Leukoc Biol. 2004. PMID: 14726499
-
Mechanotransduction in leukocyte activation: a review.Biorheology. 2007;44(4):221-49. Biorheology. 2007. PMID: 18094448 Review.
Cited by
-
Actin polymerization stabilizes α4β1 integrin anchors that mediate monocyte adhesion.J Cell Biol. 2012 Apr 2;197(1):115-29. doi: 10.1083/jcb.201107140. J Cell Biol. 2012. PMID: 22472442 Free PMC article.
-
Anti-metastatic functions of type 1 interferons: Foundation for the adjuvant therapy of cancer.Cytokine. 2017 Jan;89:4-11. doi: 10.1016/j.cyto.2016.01.010. Epub 2016 Jan 25. Cytokine. 2017. PMID: 26822709 Free PMC article. Review.
-
Effects of Mechanical Stress on Endothelial Cells In Situ and In Vitro.Int J Mol Sci. 2023 Nov 20;24(22):16518. doi: 10.3390/ijms242216518. Int J Mol Sci. 2023. PMID: 38003708 Free PMC article. Review.
-
Effects of Transient Exposure to High Shear on Neutrophil Rolling Behavior.Cell Mol Bioeng. 2018 Aug;11(4):279-290. doi: 10.1007/s12195-018-0533-z. Epub 2018 Jun 1. Cell Mol Bioeng. 2018. PMID: 31372187 Free PMC article.
-
Coordinated Mechanosensitivity of Membrane Rafts and Focal Adhesions.Cell Mol Bioeng. 2012 Jun 1;5(2):143-154. doi: 10.1007/s12195-012-0225-z. Cell Mol Bioeng. 2012. PMID: 23487555 Free PMC article.
References
-
- Sugihara-Seki M., Schmid-Schönbein G.W. The fluid shear stress distribution on the membrane of leukocytes in the microcirculation. J. Biomech. Eng. 2003;125:628–638. - PubMed
-
- Shen Z., Lipowsky H.H. Image enhancement of the in vivo leukocyte-endothelium contact zone using optical sectioning microscopy. Ann. Biomed. Eng. 1997;25:521–535. - PubMed
-
- Ohashi K.L., Tung D.K.-L., Wilson J., Zweifach B.W., Schmid-Schönbein G.W. Transvascular and interstitial migration of neutrophils in rat mesentery. Microcirculation. 1996;3:199–210. - PubMed
-
- McIntire L.V., Dewitz T.S., Martin R.R. Mechanical trauma effects on leukocytes. Trans. Am. Soc. Artif. Intern. Organs. 1976;22:444–449. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

