Life and times of a cellular bleb
- PMID: 17921219
- PMCID: PMC2242777
- DOI: 10.1529/biophysj.107.113605
Life and times of a cellular bleb
Abstract
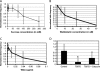
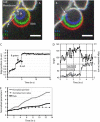
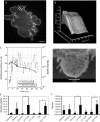
Blebs are spherical cellular protrusions that occur in many physiological situations. Two distinct phases make up the life of a bleb, each of which have their own biology and physics: expansion, which lasts approximately 30 s, and retraction, which lasts approximately 2 min. We investigate these phases using optical microscopy and simple theoretical concepts, seeking information on blebbing itself, and on cytomechanics in general. We show that bleb nucleation depends on pressure, membrane-cortex adhesion energy, and membrane tension, and test this experimentally. Bleb growth occurs through a combination of bulk flow of lipids and delamination from the cell cortex via the formation and propagation of tears. In extreme cases, this can give rise to a traveling wave around the cell periphery, known as "circus movement." When growth stalls, an actin cortex reforms under the bleb membrane, and retraction starts, driven by myosin-II. Using flicker spectroscopy, we find that retracting blebs are fivefold more rigid than expanding blebs, an increase entirely explained by the properties of the newly formed cortical actin mesh. Finally, using artificially nucleated blebs as pressure sensors, we show that cells rounded up in mitosis possess a substantial intracellular pressure.
Figures




References
-
- Paluch E., Sykes C., Prost J., Bornens M. Dynamic modes of the cortical actomyosin gel during cell locomotion and division. Trends Cell Biol. 2006;16:5–10. - PubMed
-
- Trinkaus J.P. Surface activity and locomotion of Fundulus deep cells during blastula and gastrula stages. Dev. Biol. 1973;30:69–103. - PubMed
-
- Friedl P., Wolf K. Tumor-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer. 2003;3:362–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

