A glycosyltransferase with a length-controlling activity as a mechanism to regulate the size of polysaccharides
- PMID: 17921247
- PMCID: PMC2034269
- DOI: 10.1073/pnas.0708025104
A glycosyltransferase with a length-controlling activity as a mechanism to regulate the size of polysaccharides
Abstract
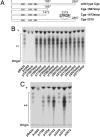
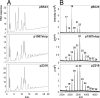
Cyclic beta-1,2-glucans (CbetaG) are osmolyte homopolysaccharides with a cyclic beta-1,2-backbone of 17-25 glucose residues present in the periplasmic space of several bacteria. Initiation, elongation, and cyclization, the three distinctive reactions required for building the cyclic structure, are catalyzed by the same protein, the CbetaG synthase. The initiation activity catalyzes the transference of the first glucose from UDP-glucose to a yet-unidentified amino acid residue in the same protein. Elongation proceeds by the successive addition of glucose residues from UDP-glucose to the nonreducing end of the protein-linked beta-1,2-oligosaccharide intermediate. Finally, the protein-linked intermediate is cyclized, and the cyclic glucan is released from the protein. These reactions do not explain, however, the mechanism by which the number of glucose residues in the cyclic structure is controlled. We now report that control of the degree of polymerization (DP) is carried out by a beta-1,2-glucan phosphorylase present at the CbetaG synthase C-terminal domain. This last activity catalyzes the phosphorolysis of the beta-1,2-glucosidic bond at the nonreducing end of the linear protein-linked intermediate, releasing glucose 1-phosphate. The DP is thus regulated by this "length-controlling" phosphorylase activity. To our knowledge, this is the first description of a control of the DP of homopolysaccharides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



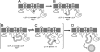
References
-
- Bohin JP. FEMS Microbiol Lett. 2000;186:11–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

