Control of the transcription of a short gene encoding a cyclic peptide in Streptococcus thermophilus: a new quorum-sensing system?
- PMID: 17921293
- PMCID: PMC2168622
- DOI: 10.1128/JB.01057-07
Control of the transcription of a short gene encoding a cyclic peptide in Streptococcus thermophilus: a new quorum-sensing system?
Abstract
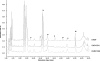
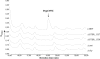
Gram-positive bacteria secrete a variety of peptides that are often subjected to posttranslational modifications and that are either antimicrobials or pheromones involved in bacterial communication. Our objective was to identify peptides secreted by Streptococcus thermophilus, a nonpathogenic bacterium widely used in dairy technology in association with other bacteria, and to understand their potential roles in cell-cell communication. Using reverse-phase liquid chromatography, mass spectrometry, and Edman sequencing, we analyzed the culture supernatants of three S. thermophilus strains (CNRZ1066, LMG18311, and LMD-9) grown in a medium containing no peptides. We identified several peptides in the culture supernatants, some of them found with the three strains while others were specific to the LMD-9 strain. We focused our study on a new modified peptide secreted by S. thermophilus LMD-9 and designated Pep1357C. This peptide contains 9 amino acids and lost 2 Da in a posttranslational modification, most probably a dehydrogenation, leading to a linkage between the Lys2 and Trp6 residues. Production of Pep1357C and transcription of its encoding gene depend on both the medium composition and the growth phase. Furthermore, we demonstrated that transcription of the gene coding for Pep1357C is drastically decreased in mutants inactivated for the synthesis of a short hydrophobic peptide, a transcriptional regulator, or the oligopeptide transport system. Taken together, our results led us to deduce that the transcription of the Pep1357C-encoding gene is controlled by a new quorum-sensing system.
Figures
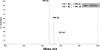

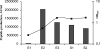


References
-
- Alloing, G., P. de Philip, and J. P. Claverys. 1994. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of the gram-positive Streptococcus pneumoniae. J. Mol. Biol. 241:44-58. - PubMed
-
- Ansaldi, M., D. Marolt, T. Stebe, I. Mandic-Mulec, and D. Dubnau. 2002. Specific activation of the Bacillus quorum-sensing systems by isoprenylated pheromone variants. Mol. Microbiol. 44:1561-1573. - PubMed
-
- Arakawa, K., F. Sugino, K. Kodama, T. Ishii, and H. Kinashi. 2005. Cyclization mechanism for the synthesis of macrocyclic antibiotic lankacidin in Streptomyces rochei. Chem. Biol. 12:249-256. - PubMed
-
- Archer, D. L. 1996. Preservation microbiology and safety: evidence that stress enhances virulence and triggers adaptive mutations. Trends Food Sci. Technol. 7:91-95.
-
- Bassler, B. L. 2002. Small talk. Cell-to-cell communication in bacteria. Cell 109:421-424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

