The yydFGHIJ operon of Bacillus subtilis encodes a peptide that induces the LiaRS two-component system
- PMID: 17921301
- PMCID: PMC2168931
- DOI: 10.1128/JB.01181-07
The yydFGHIJ operon of Bacillus subtilis encodes a peptide that induces the LiaRS two-component system
Abstract
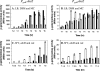
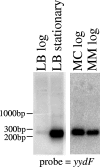
The Bacillus subtilis LiaRS two-component system (TCS) responds to perturbations of the cell envelope induced by lipid II-interacting antibiotics, such as vancomycin, ramoplanin, nisin, and bacitracin. Here, we characterize Tn7-generated mutations that induce the liaRS TCS. In addition to insertions in liaF, a known negative regulator of the LiaRS TCS, we identified two disruptions in the last two genes of the yydFGHIJ operon. This operon is predicted to encode a 49-amino-acid peptide (YydF), a modification enzyme (YydG), a membrane-embedded protease (YydH), and an ATP-binding cassette (ABC) transporter (YydIJ). Genome sequence comparisons suggest that the yydFGHIJ operon may have been acquired by horizontal transfer. Inactivation of the YydIJ transporter resulted in increased expression from the LiaR-dependent P(liaI) promoter only in the presence of the yydFGH genes. Cells harboring the complete yydFGHIJ operon induced LiaR activity in cocultured cells lacking either this transporter or the complete operon. These results suggest that this operon is involved in the synthesis and export of a modified peptide (YydF*) that elicits cell envelope stress sensed by the LiaRS TCS.
Figures



Similar articles
-
In-depth profiling of the LiaR response of Bacillus subtilis.J Bacteriol. 2010 Sep;192(18):4680-93. doi: 10.1128/JB.00543-10. Epub 2010 Jul 16. J Bacteriol. 2010. PMID: 20639339 Free PMC article.
-
Antibiotic-inducible promoter regulated by the cell envelope stress-sensing two-component system LiaRS of Bacillus subtilis.Antimicrob Agents Chemother. 2004 Aug;48(8):2888-96. doi: 10.1128/AAC.48.8.2888-2896.2004. Antimicrob Agents Chemother. 2004. PMID: 15273097 Free PMC article.
-
Regulation of LiaRS-dependent gene expression in bacillus subtilis: identification of inhibitor proteins, regulator binding sites, and target genes of a conserved cell envelope stress-sensing two-component system.J Bacteriol. 2006 Jul;188(14):5153-66. doi: 10.1128/JB.00310-06. J Bacteriol. 2006. PMID: 16816187 Free PMC article.
-
Low phosphatase activity of LiaS and strong LiaR-DNA affinity explain the unusual LiaS to LiaR in vivo stoichiometry.BMC Microbiol. 2020 Apr 29;20(1):104. doi: 10.1186/s12866-020-01796-6. BMC Microbiol. 2020. PMID: 32349670 Free PMC article.
-
LiaRS-dependent gene expression is embedded in transition state regulation in Bacillus subtilis.Microbiology (Reading). 2007 Aug;153(Pt 8):2530-2540. doi: 10.1099/mic.0.2007/006817-0. Microbiology (Reading). 2007. PMID: 17660417
Cited by
-
Radical S-Adenosylmethionine Enzymes Involved in RiPP Biosynthesis.Biochemistry. 2017 Oct 10;56(40):5229-5244. doi: 10.1021/acs.biochem.7b00771. Epub 2017 Sep 22. Biochemistry. 2017. PMID: 28895719 Free PMC article. Review.
-
Metabolome Analysis of Constituents in Membrane Vesicles for Clostridium thermocellum Growth Stimulation.Microorganisms. 2021 Mar 13;9(3):593. doi: 10.3390/microorganisms9030593. Microorganisms. 2021. PMID: 33805707 Free PMC article.
-
Mechanism of Action of Ribosomally Synthesized and Post-Translationally Modified Peptides.Chem Rev. 2022 Sep 28;122(18):14722-14814. doi: 10.1021/acs.chemrev.2c00210. Epub 2022 Sep 1. Chem Rev. 2022. PMID: 36049139 Free PMC article. Review.
-
Peptide Epimerization Machineries Found in Microorganisms.Front Microbiol. 2018 Feb 6;9:156. doi: 10.3389/fmicb.2018.00156. eCollection 2018. Front Microbiol. 2018. PMID: 29467749 Free PMC article. Review.
-
Radical SAM Enzymes in the Biosynthesis of Ribosomally Synthesized and Post-translationally Modified Peptides (RiPPs).Front Chem. 2017 Nov 8;5:87. doi: 10.3389/fchem.2017.00087. eCollection 2017. Front Chem. 2017. PMID: 29167789 Free PMC article. Review.
References
-
- Butcher, B. G., and J. D. Helmann. 2006. Identification of Bacillus subtilis σW-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by bacilli. Mol. Microbiol. 60:765-782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

