Unusual starch degradation pathway via cyclodextrins in the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus strain 7324
- PMID: 17921308
- PMCID: PMC2168605
- DOI: 10.1128/JB.01136-07
Unusual starch degradation pathway via cyclodextrins in the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus strain 7324
Abstract
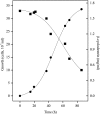
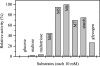
The hyperthermophilic archaeon Archaeoglobus fulgidus strain 7324 has been shown to grow on starch and sulfate and thus represents the first sulfate reducer able to degrade polymeric sugars. The enzymes involved in starch degradation to glucose 6-phosphate were studied. In extracts of starch-grown cells the activities of the classical starch degradation enzymes, alpha-amylase and amylopullulanase, could not be detected. Instead, evidence is presented here that A. fulgidus utilizes an unusual pathway of starch degradation involving cyclodextrins as intermediates. The pathway comprises the combined action of an extracellular cyclodextrin glucanotransferase (CGTase) converting starch to cyclodextrins and the intracellular conversion of cyclodextrins to glucose 6-phosphate via cyclodextrinase (CDase), maltodextrin phosphorylase (Mal-P), and phosphoglucomutase (PGM). These enzymes, which are all induced after growth on starch, were characterized. CGTase catalyzed the conversion of starch to mainly beta-cyclodextrin. The gene encoding CGTase was cloned and sequenced and showed highest similarity to a glucanotransferase from Thermococcus litoralis. After transport of the cyclodextrins into the cell by a transport system to be defined, these molecules are linearized via a CDase, catalyzing exclusively the ring opening of the cyclodextrins to the respective maltooligodextrins. These are degraded by a Mal-P to glucose 1-phosphate. Finally, PGM catalyzes the conversion of glucose 1-phosphate to glucose 6-phosphate, which is further degraded to pyruvate via the modified Embden-Meyerhof pathway.
Figures







Similar articles
-
Sugar utilization in the hyperthermophilic, sulfate-reducing archaeon Archaeoglobus fulgidus strain 7324: starch degradation to acetate and CO2 via a modified Embden-Meyerhof pathway and acetyl-CoA synthetase (ADP-forming).Arch Microbiol. 2001 Nov;176(5):329-38. doi: 10.1007/s002030100330. Arch Microbiol. 2001. PMID: 11702074
-
Transcriptional and biochemical analysis of starch metabolism in the hyperthermophilic archaeon Pyrococcus furiosus.J Bacteriol. 2006 Mar;188(6):2115-25. doi: 10.1128/JB.188.6.2115-2125.2006. J Bacteriol. 2006. PMID: 16513741 Free PMC article.
-
Maltose metabolism in the hyperthermophilic archaeon Thermococcus litoralis: purification and characterization of key enzymes.J Bacteriol. 1999 Jun;181(11):3358-67. doi: 10.1128/JB.181.11.3358-3367.1999. J Bacteriol. 1999. PMID: 10348846 Free PMC article.
-
Cyclodextrin glucanotransferase: fundamentals and biotechnological implications.Appl Microbiol Biotechnol. 2023 Oct;107(19):5899-5907. doi: 10.1007/s00253-023-12708-9. Epub 2023 Aug 7. Appl Microbiol Biotechnol. 2023. PMID: 37548666 Review.
-
[The product specificity evolution of cyclodextrin glucanotransferase: problems and challenges].Sheng Wu Gong Cheng Xue Bao. 2007 Mar;23(2):181-8. Sheng Wu Gong Cheng Xue Bao. 2007. PMID: 17460885 Review. Chinese.
Cited by
-
Sequence fingerprints of enzyme specificities from the glycoside hydrolase family GH57.Extremophiles. 2012 May;16(3):497-506. doi: 10.1007/s00792-012-0449-9. Epub 2012 Apr 22. Extremophiles. 2012. PMID: 22527043
-
Role of maltogenic amylase and pullulanase in maltodextrin and glycogen metabolism of Bacillus subtilis 168.J Bacteriol. 2009 Aug;191(15):4835-44. doi: 10.1128/JB.00176-09. Epub 2009 May 22. J Bacteriol. 2009. PMID: 19465663 Free PMC article.
-
α-Amylase: an enzyme specificity found in various families of glycoside hydrolases.Cell Mol Life Sci. 2014 Apr;71(7):1149-70. doi: 10.1007/s00018-013-1388-z. Epub 2013 Jun 27. Cell Mol Life Sci. 2014. PMID: 23807207 Free PMC article. Review.
-
Acute and Delayed Effects of Stress Eliciting Post-Traumatic Stress-Like Disorder Differentially Alters Fecal Microbiota Composition in a Male Mouse Model.Front Cell Infect Microbiol. 2022 Mar 1;12:810815. doi: 10.3389/fcimb.2022.810815. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35300376 Free PMC article.
-
Complete genome sequence analysis of Archaeoglobus fulgidus strain 7324 (DSM 8774), a hyperthermophilic archaeal sulfate reducer from a North Sea oil field.Stand Genomic Sci. 2017 Dec 16;12:79. doi: 10.1186/s40793-017-0296-5. eCollection 2017. Stand Genomic Sci. 2017. PMID: 29270248 Free PMC article.
References
-
- Akutsu, J., Z. Zhang, M. Tsujimura, M. Sasaki, M. Yohda, and Y. Kawarabayasi. 2005. Characterization of a thermostable enzyme with phosphomannomutase/phosphoglucomutase activities from the hyperthermophilic archaeon Pyrococcus horikoshii OT3. J. Biochem. 138:159-166. - PubMed
-
- Albers, S. V., J. L. Van de Vossenberg, A. J. Driessen, and W. N. Konings. 2001. Bioenergetics and solute uptake under extreme conditions. Extremophiles 5:285-294. - PubMed
-
- Bender, H. 1977. Cyclodextrin glucanotransferase from Klebsiella pneumoniae. 2. Significance of the enzyme for the metabolism of cyclodextrins by Klebsiella pneumoniae M 5. Arch. Microbiol. 113:49-56. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

