Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato
- PMID: 17921340
- PMCID: PMC2151702
- DOI: 10.1104/pp.107.106500
Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato
Abstract
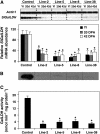
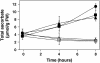
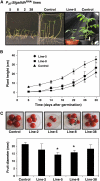
L-Galactono-1,4-lactone dehydrogenase (EC 1.3.2.3) catalyzes the last step in the main pathway of vitamin C (L-ascorbic acid) biosynthesis in higher plants. In this study, we first characterized the spatial and temporal expression of SlGalLDH in several organs of tomato (Solanum lycopersicum) plants in parallel with the ascorbate content. P(35S):Slgalldh(RNAi) silenced transgenic tomato lines were then generated using an RNAi strategy to evaluate the effect of any resulting modification of the ascorbate pool on plant and fruit development. In all P(35S):Slgalldh(RNAi) plants with reduced SlGalLDH transcript and activity, plant growth rate was decreased. Plants displaying the most severe effects (dwarf plants with no fruit) were excluded from further analysis. The most affected lines studied exhibited up to an 80% reduction in SlGalLDH activity and showed a strong reduction in leaf and fruit size, mainly as a consequence of reduced cell expansion. This was accompanied by significant changes in mitochondrial function and altered ascorbate redox state despite the fact that the total ascorbate content remained unchanged. By using a combination of transcriptomic and metabolomic approaches, we further demonstrated that several primary, like the tricarboxylic acid cycle, as well as secondary metabolic pathways related to stress response were modified in leaves and fruit of P(35S):Slgalldh(RNAi) plants. When taken together, this work confirms the complexity of ascorbate regulation and its link with plant metabolism. Moreover, it strongly suggests that, in addition to ascorbate synthesis, GalLDH could play an important role in the regulation of cell growth-related processes in plants.
Figures



References
-
- Agius F, Gonzalez-Lamonthe R, Caballero JL, Munoz-Blanco L, Botella MA, Valpuesta V (2003) Engineering increased vitamin C levels in plants by over-expression of a D-galacturonic acid reductase. Nat Biotechnol 21 177–181 - PubMed
-
- Alba R, Fei Z, Payton P, Liu Y, Moore SL, Debbie P, Cohn J, D'Ascenzo M, Gordon JS, Rose JK, et al (2004) ESTs, cDNA microarrays, and gene expression profiling: tools for dissecting plant physiology and development. Plant J 39 697–714 - PubMed
-
- Andrews PK, Fahy DA, Foyer CH (2004) Relationships between fruit exocarp antioxidants in the tomato (Lycopersicon esculentum) high pigment-1 mutant during development. Physiol Plant 120 519–528 - PubMed
-
- Bartoli CG, Guiamet JJ, Kiddle G, Pastori GM, Di Cagno R, Theodoulou FL, Foyer CH (2005) Ascorbate content of wheat leaves is not determined by maximal L-galactono-1,4-lactone dehydrogenase (GalLDH) activity under drought stress. Plant Cell Environ 28 1073–1081
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

