Endocytosis in the shiitake mushroom Lentinula edodes and involvement of GTPase LeRAB7
- PMID: 17921351
- PMCID: PMC2168259
- DOI: 10.1128/EC.00222-07
Endocytosis in the shiitake mushroom Lentinula edodes and involvement of GTPase LeRAB7
Abstract
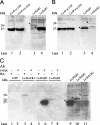
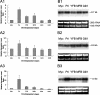
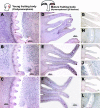
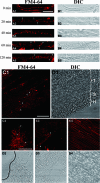
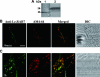
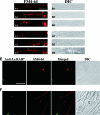
Endocytosis is the process by which substrates enter a cell without passing through the plasma membrane but rather invaginate the cell membrane and form intracellular vesicles. Rab7 regulates endocytic trafficking between early and late endosomes and between late endosomes and lysosomes. LeRab7 in Lentinula edodes is strongly homologous to Rab7 in Homo sapiens. Receptors for activated C kinase-1 (LeRACK1) and Rab5 GTPase (LeRAB5) were isolated as interacting partners of LeRab7, and the interactions were confirmed by in vivo and in vitro protein interaction assays. The three genes showed differential expression in the various developmental stages of the mushroom. In situ hybridization showed that the three transcripts were localized in regions of active growth, such as the outer region of trama cells, and the subhymenium of the hymenophore of mature fruiting bodies and the prehymenophore of young fruiting bodies. The existence of endocytosis in the mycelium and hymenophores was confirmed by the internalization of FM4-64. LeRAB7 was partially colocalized with the AM4-64 and was located in the late endocytic pathway. This is the first report of the presence of endocytosis in homobasidiomycetes. LeRAB7, LeRAB5, and LeRACK1 may contribute to the growth of L. edodes and cell differentiation in hymenophores.
Figures

Similar articles
-
Identification by RNA fingerprinting of genes differentially expressed during the development of the basidiomycete Lentinula edodes.Mol Gen Genet. 2000 Jan;262(6):977-90. doi: 10.1007/pl00008666. Mol Gen Genet. 2000. PMID: 10660059
-
The fruiting-specific Le.flp1 gene, encoding a novel fungal fasciclin-like protein, of the basidiomycetous mushroom Lentinula edodes.Curr Genet. 2007 Jun;51(6):367-75. doi: 10.1007/s00294-007-0133-2. Epub 2007 May 3. Curr Genet. 2007. PMID: 17476508
-
Specific distribution in homobasidiomycete hymenophores of the transcripts of Ras protein and G-protein alpha-subunit genes.FEMS Microbiol Lett. 2005 Jan 1;242(1):169-75. doi: 10.1016/j.femsle.2004.11.025. FEMS Microbiol Lett. 2005. PMID: 15621434
-
Regulation of membrane transport through the endocytic pathway by rabGTPases.Mol Membr Biol. 1999 Jan-Mar;16(1):81-7. doi: 10.1080/096876899294797. Mol Membr Biol. 1999. PMID: 10332741 Review.
-
Endocytic Rabs in membrane trafficking and signaling.Biol Chem. 2014 Mar;395(3):327-33. doi: 10.1515/hsz-2013-0258. Biol Chem. 2014. PMID: 24158421 Review.
Cited by
-
The curtain model as an alternative and complementary to the classic turgor concept of filamentous fungi.Arch Microbiol. 2025 Feb 20;207(3):65. doi: 10.1007/s00203-025-04271-w. Arch Microbiol. 2025. PMID: 39979668 Review.
References
-
- Atkinson, H. A., A. Daniels, and N. D. Read. 2002. Live-cell imaging of endocytosis during conidial germination in the rice blast fungus, Magnaporthe grisea. Fungal Genet. Biol. 37:233-244. - PubMed
-
- Ayscough, K. R. 2005. Defining protein modules for endocytosis. Cell 123:305-320. - PubMed
-
- Brabec, M., D. Blaas, and D. Fuchs. 2006. Wortmannin delays transfer of human rhinovirus serotype 2 to late endocytic compartments. Biochem. Biophys. Res. Commun. 348:741-749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

