Dp71, utrophin and beta-dystroglycan expression and distribution in PC12/L6 cell cocultures
- PMID: 17921863
- PMCID: PMC2580073
- DOI: 10.1097/WNR.0b013e3282f0e42d
Dp71, utrophin and beta-dystroglycan expression and distribution in PC12/L6 cell cocultures
Abstract
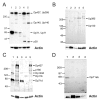
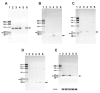
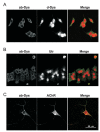
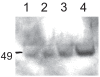
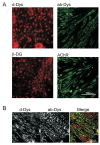
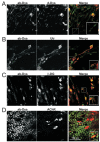
Function of dystrophin Dp71 isoforms is unknown but seems related to neurite outgrowth and synapse formation. To evaluate Dp71 role in myoneural synapses, we established a coculture model using PC12 cells and L6 myotubes and analyzed expression and localization of Dp71 and related proteins, utrophin and beta-dystroglycan, in PC12 cells. Confocal microscopy showed Dp71d isoform in PC12 nuclei, golgi-complex-like and endoplasmic reticulum-like structures, whereas Dp71ab concentrates at neurite tips and cytoplasm, colocalizing with beta-dystroglycan, utrophin, synaptophysin and acetylcholine receptors. Evidences suggest that Dp71ab isoform, unlike Dp71d, may take part in neurite-related processes. This is the first work on Dp and members of Dp-associated protein complex roles in a cell-line based coculturing system, which may be useful in determining Dp71 isoforms associations.
Figures



Similar articles
-
Differential expression and subcellular distribution of dystrophin Dp71 isoforms during differentiation process.Neuroscience. 2003;118(4):957-66. doi: 10.1016/s0306-4522(03)00063-0. Neuroscience. 2003. PMID: 12732241
-
Identification of Dp71 Isoforms Expressed in PC12 Cells: Subcellular Localization and Colocalization with β-Dystroglycan and α1-Syntrophin.J Mol Neurosci. 2016 Feb;58(2):201-9. doi: 10.1007/s12031-015-0657-8. Epub 2015 Sep 28. J Mol Neurosci. 2016. PMID: 26411569
-
Molecular heterogeneity of the dystrophin-associated protein complex in the mouse kidney nephron: differential alterations in the absence of utrophin and dystrophin.Cell Tissue Res. 2005 Feb;319(2):299-313. doi: 10.1007/s00441-004-0999-y. Epub 2004 Nov 25. Cell Tissue Res. 2005. PMID: 15565469
-
Regulation and functional significance of utrophin expression at the mammalian neuromuscular synapse.Microsc Res Tech. 2000 Apr 1;49(1):90-100. doi: 10.1002/(SICI)1097-0029(20000401)49:1<90::AID-JEMT10>3.0.CO;2-L. Microsc Res Tech. 2000. PMID: 10757882 Review.
-
Dystrophin-associated proteins and synapse formation: is alpha-dystroglycan the agrin receptor?Cell. 1994 Jun 3;77(5):617-9. doi: 10.1016/0092-8674(94)90045-0. Cell. 1994. PMID: 8205610 Review. No abstract available.
Cited by
-
Human Dystrophin Dp71ab Enhances the Proliferation of Myoblasts Across Species But Not Human Nonmyoblast Cells.Front Cell Dev Biol. 2022 Apr 25;10:877612. doi: 10.3389/fcell.2022.877612. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35547811 Free PMC article.
References
-
- Acosta R, et al. Dystrophin Dp71 is required for neurite outgrowth in PC12 cells. Exp Cell Res. 2003;296:265–275. - PubMed
-
- Adams ME. Two forms of mouse syntrophin, a 58 kd dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron. 1993;11:531–540. - PubMed
-
- Apel ED. Rapsyn is required for MuSK signalling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18:623–635. - PubMed
-
- Avila AM. Differential regulation of nicotinic acetylcholine receptors in PC12 cells by nicotine and nerve growth factor. Mol Pharmacol. 2003;64:974–986. - PubMed
-
- Bardoni A. Loss of Dp140 regulatory sequences is associated with cognitive impairment in dystrophinopathies. Neuromuscul Disord. 2000;10:194–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

