Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations
- PMID: 17922008
- PMCID: PMC2838615
- DOI: 10.1038/nn1976
Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations
Abstract
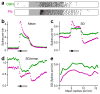
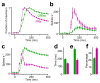
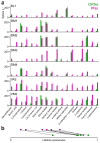
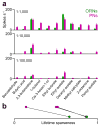
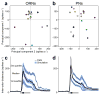
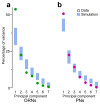
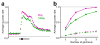
Here we describe several fundamental principles of olfactory processing in the Drosophila melanogaster antennal lobe (the analog of the vertebrate olfactory bulb), through the systematic analysis of input and output spike trains of seven identified glomeruli. Repeated presentations of the same odor elicit more reproducible responses in second-order projection neurons (PNs) than in their presynaptic olfactory receptor neurons (ORNs). PN responses rise and accommodate rapidly, emphasizing odor onset. Furthermore, weak ORN inputs are amplified in the PN layer but strong inputs are not. This nonlinear transformation broadens PN tuning and produces more uniform distances between odor representations in PN coding space. In addition, portions of the odor response profile of a PN are not systematically related to their direct ORN inputs, which probably indicates the presence of lateral connections between glomeruli. Finally, we show that a linear discriminator classifies odors more accurately using PN spike trains than using an equivalent number of ORN spike trains.
Figures


Comment in
-
A step toward optimal coding in olfaction.Nat Neurosci. 2007 Nov;10(11):1342-3. doi: 10.1038/nn1107-1342. Nat Neurosci. 2007. PMID: 17965649 No abstract available.
References
-
- Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. - PubMed
-
- Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004;5:263–78. - PubMed
-
- Laissue PP, et al. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J Comp Neurol. 1999;405:543–52. - PubMed
-
- Hallem EA, Carlson JR. The odor coding system of Drosophila. Trends Genet. 2004;20:453–9. - PubMed
-
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–47. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

