Phosphatase and tensin homologue deleted on chromosome ten (PTEN) as a molecular target in lung epithelial wound repair
- PMID: 17922022
- PMCID: PMC2189995
- DOI: 10.1038/sj.bjp.0707501
Phosphatase and tensin homologue deleted on chromosome ten (PTEN) as a molecular target in lung epithelial wound repair
Abstract
Background and purpose: Epithelial injury contributes to lung pathogenesis. Our work and that of others have identified the phosphoinositide-3 kinase (PI3K)/Akt pathway as a vital component of survival in lung epithelia. Therefore, we hypothesized that pharmacological inhibition of PTEN, a major suppressor of this pathway, would enhance wound closure and restore lung epithelial monolayer integrity following injury.
Experimental approach: We evaluated the ability of two bisperoxovanadium derivatives, bpV(phen) and bpV(pic), in differentiated primary human airway epithelia and BEAS2B cultures for their ability to inhibit PTEN, activate the PI3K/Akt pathway and restore epithelial monolayer integrity following mechanical injury.
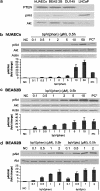
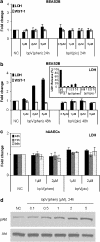
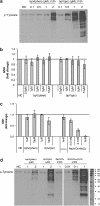
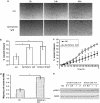
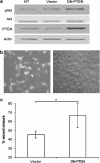
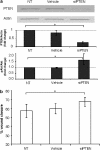
Key results: BpV(phen) and bpV(pic) induced Akt phosphorylation in primary and BEAS2B cells in a dose and time dependent manner. Minimal toxicity was observed as measured by lactate dehydrogenase (LDH) release. To verify that Akt phosphorylation is specifically induced by PTEN inhibition, the PTEN positive cell line, DU145, and two PTEN negative cell lines, LNCaP and PC3, were examined. PTEN positive cells demonstrated a dose responsive increase in Akt phosphorylation whereas PTEN negative cells showed no response indicating that bpV(phen) directly suppresses PTEN without affecting auxiliary pathways. Next, we observed that exposure to either compound resulted in accelerated wound closure following mechanical injury. Similar effects were observed after transfection with a dominant negative isoform of PTEN and PTEN specific siRNA.
Conclusions and implications: From these studies, we conclude that PTEN is a valid target for future studies directed at restoring epithelial barrier function after lung injury.
Figures


Comment in
-
PTEN: a promising pharmacological target to enhance epithelial wound healing.Br J Pharmacol. 2007 Dec;152(8):1141-4. doi: 10.1038/sj.bjp.0707503. Epub 2007 Oct 8. Br J Pharmacol. 2007. PMID: 17922021 Free PMC article.
Similar articles
-
Inhibition of the phosphatase PTEN protects mice against oleic acid-induced acute lung injury.Br J Pharmacol. 2009 Jan;156(1):189-200. doi: 10.1111/j.1476-5381.2008.00020.x. Br J Pharmacol. 2009. PMID: 19134000 Free PMC article.
-
PTEN: a promising pharmacological target to enhance epithelial wound healing.Br J Pharmacol. 2007 Dec;152(8):1141-4. doi: 10.1038/sj.bjp.0707503. Epub 2007 Oct 8. Br J Pharmacol. 2007. PMID: 17922021 Free PMC article.
-
PTEN inhibition improves wound healing in lung epithelia through changes in cellular mechanics that enhance migration.Am J Physiol Lung Cell Mol Physiol. 2012 Feb 1;302(3):L287-99. doi: 10.1152/ajplung.00037.2011. Epub 2011 Oct 28. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22037358 Free PMC article.
-
Biological role of phosphatase PTEN in cancer and tissue injury healing.Front Biosci. 2002 May 1;7:e245-51. doi: 10.2741/tsugawa. Front Biosci. 2002. PMID: 11991859 Review.
-
PTEN modulators: a patent review.Expert Opin Ther Pat. 2013 May;23(5):569-80. doi: 10.1517/13543776.2013.768985. Epub 2013 Feb 5. Expert Opin Ther Pat. 2013. PMID: 23379765 Free PMC article. Review.
Cited by
-
Lung inflammation, injury, and proliferative response after repetitive particulate hexavalent chromium exposure.Environ Health Perspect. 2009 Dec;117(12):1896-902. doi: 10.1289/ehp.0900715. Epub 2009 Aug 19. Environ Health Perspect. 2009. PMID: 20049209 Free PMC article.
-
mir-106a regulates cell proliferation and apoptosis of colon cancer cells through targeting the PTEN/PI3K/AKT signaling pathway.Oncol Lett. 2018 Mar;15(3):3197-3201. doi: 10.3892/ol.2017.7715. Epub 2017 Dec 29. Oncol Lett. 2018. PMID: 29435057 Free PMC article.
-
MicroRNA-26a-3p rescues depression-like behaviors in male rats via preventing hippocampal neuronal anomalies.J Clin Invest. 2021 Aug 16;131(16):e148853. doi: 10.1172/JCI148853. J Clin Invest. 2021. PMID: 34228643 Free PMC article.
-
Lipopolysaccharide induces lung fibroblast proliferation through Toll-like receptor 4 signaling and the phosphoinositide3-kinase-Akt pathway.PLoS One. 2012;7(4):e35926. doi: 10.1371/journal.pone.0035926. Epub 2012 Apr 26. PLoS One. 2012. PMID: 22563417 Free PMC article.
-
A new role for PTEN in regulating transient receptor potential canonical channel 6-mediated Ca2+ entry, endothelial permeability, and angiogenesis.J Biol Chem. 2010 Oct 22;285(43):33082-33091. doi: 10.1074/jbc.M110.142034. Epub 2010 Aug 12. J Biol Chem. 2010. PMID: 20705603 Free PMC article.
References
-
- Bao S, Wang Y, Sweeney P, Chaudhuri A, Doseff AI, Marsh CB, et al. Keratinocyte growth factor induces Akt kinase activity and inhibits Fas-mediated apoptosis in A549 lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L36–L42. - PubMed
-
- Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, et al. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. - PubMed
-
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, et al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. - PubMed
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
-
- Cerovac Z, Ban J, Morinville A, Yaccato K, Shaver A, Maysinger D. Activation of MAPK by potassium bisperoxo(1,10-phenanthroline)oxovanadate (V) Neurochem Int. 1999;34:337–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

