Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors
- PMID: 17922185
- PMCID: PMC3081690
- DOI: 10.1007/s10549-007-9774-6
Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors
Abstract
Purpose: Aromatase inhibitors (AIs) are increasingly used as adjuvant treatment of postmenopausal women with hormone receptor-positive breast cancer. AIs are commonly associated with musculoskeletal symptoms. The primary objective of this study was to describe the musculoskeletal symptoms that developed in the first 100 subjects enrolled who had at least 6 months follow-up.
Methods: Women with early stage hormone receptor-positive breast cancer were recruited into a multicenter randomized clinical trial to study the pharmacogenomics of two AIs, exemestane, and letrozole. Patients completed the Health Assessment Questionnaire (HAQ) and Visual Analog Scale (VAS) at baseline, 1, 3, 6, and 12 months to assess changes in function and pain, respectively. Patients were referred for evaluation by a rheumatologist if their HAQ and/or VAS scores exceeded a predefined threshold.
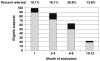
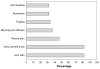
Results: Forty-four of 97 eligible patients (45.4%) met criteria for rheumatologic referral. Three patients were ineligible because of elevated baseline HAQ (2) and failure to initiate AI therapy (1). No baseline characteristics were significantly associated with referral. Median time to onset of symptoms was 1.6 months (range 0.4-10 months). Clinical and laboratory evaluation of patients evaluated by rheumatology suggested that the majority developed either non-inflammatory musculoskeletal symptoms or inflammation localized to tenosynovial structures. Thirteen patients discontinued AI therapy because of musculoskeletal toxicity after a median 6.1 months (range 2.2-13 months).
Conclusions: Musculoskeletal side effects were common in AI-treated patients, resulting in therapy discontinuation in more than 10% of patients. There are no identifiable pre-therapy indicators of risk, and the etiology remains elusive.
Figures
References
-
- Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet. 2002;359:2131–2139. - PubMed
-
- Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. - PubMed
-
- Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. - PubMed
-
- Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25:486–492. - PubMed
-
- Boccardo F, Rubagotti A, Puntoni M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole trial. J Clin Oncol. 2005;23:5138–5147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

