Role of the transmembrane domain of glycoprotein IX in assembly of the glycoprotein Ib-IX complex
- PMID: 17922811
- PMCID: PMC2670928
- DOI: 10.1111/j.1538-7836.2007.02785.x
Role of the transmembrane domain of glycoprotein IX in assembly of the glycoprotein Ib-IX complex
Abstract
Background: The glycoprotein (GP) Ib-IX complex is critically involved in platelet adhesion to von Willebrand factor and in the initial step of platelet activation. How this complex is assembled is not clear. We previously showed that the transmembrane (TM) domains of the GPIbalpha and GPIbbeta subunits interact and participate in complex assembly.
Objectives and methods: Here, we have investigated the role of the TM and cytoplasmic domains of GPIX in assembly of the GPIb-IX complex, by analyzing the mutational effects on complex expression and assembly in transiently transfected Chinese hamster ovary cells.
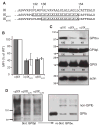
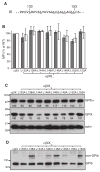
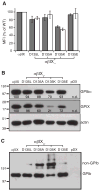
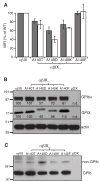
Results: Replacing the cytoplasmic domain of GPIX with a poly-alanine sequence had little effect on surface expression and structural integrity of the GPIb-IX complex. In contrast, replacing the GPIX TM domain (residues 132-153) with a poly-leucine-alanine sequence markedly disrupted complex formation of GPIX with GPIbalpha, interfered with GPIb formation, and decreased surface expression of the host complex. We further analyzed the contributions of a number of GPIX TM residues to complex formation by mutagenesis and found significant roles for Asp135 and several Leu residues.
Conclusions: The TM domain, rather than the cytoplasmic domain, of GPIX plays an important role in expression and assembly of the GPIb-IX complex by interacting with its counterparts of GPIb. These TM domains may form a parallel four-helical bundle structure in the complex.
Figures





References
-
- Berndt MC, Shen Y, Dopheide SM, Gardiner EE, Andrews RK. The vascular biology of the glycoprotein Ib-IX-V complex. Thromb Haemost. 2001;86:178–88. - PubMed
-
- Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100:2102–7. - PubMed
-
- Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, von Andrian UH, Wagner DD, Stossel TP, Hartwig JH. The clearance mechanism of chilled blood platelets. Cell. 2003;112:87–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

