Effect of CYP2C19 polymorphism on nelfinavir to M8 biotransformation in HIV patients
- PMID: 17922881
- PMCID: PMC2291390
- DOI: 10.1111/j.1365-2125.2007.03039.x
Effect of CYP2C19 polymorphism on nelfinavir to M8 biotransformation in HIV patients
Abstract
What is already known about this subject: * Nelfinavir is an HIV protease inhibitor, substrate of the transporter P-glycoprotein and metabolized via CYP2C19, CYP3A4 and CYP3A5 enzymes. * Pharmacokinetic studies have shown wide interindividual variability of nelfinavir concentrations, some of this variability perhaps caused by variant drug metabolism or transporter genes. * For CYP3A4*1B and CYP3A5*3 polymorphism, results from three studies are in agreement, showing no difference in nelfinavir concentrations between patients with these different genotypes. * However, for MDR1 and CYP2C19 polymorphism, there have been contradictory studies, showing either no impact on nelfinavir concentration or modified concentrations which could influence virological response.
What this study adds: * Patients with an *1/*2 or *2/*2 genotype for CYP2C19 had a nelfinavir to M8 biotransformation divided by 2 compared with *1/*1 patients. * No evidence of any influence of MDR1 polymorphism on nelfinavir absorption could be detected.
Aims: To evaluate the effect of CYP2C19 polymorphism on nelfinavir and M8 pharmacokinetic variability in human immunodeficiency virus-infected patients and to study the link between pharmacokinetic exposure and short-term efficacy and toxicity.
Methods: Nelfinavir (n = 120) and M8 (n = 119) concentrations were measured in 34 protease inhibitor-naive patients. Two weeks after initiating the treatment, blood samples were taken before, 1, 3 and 6 h after drug administration. Genotyping for CYP3A4, 3A5, 2C19 and MDR1 was performed. A population pharmacokinetic model was developed to describe nelfinavir-M8 concentration time-courses and to estimate interpatient variability. The influence of individual characteristics and genotypes were tested using a likelihood ratio test. Estimated mean (C(mean)), maximal (C(max)) and trough (C(trough)) nelfinavir and M8 concentrations were correlated to short-term virological efficacy and tolerance using Spearman nonparametric correlation tests.
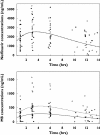
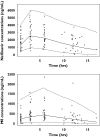
Results: A one-compartment model with first-order absorption, elimination and metabolism to M8 best described nelfinavir data. M8 was modelled by an additional compartment. Mean pharmacokinetic estimates and the corresponding intersubject variabilities were: absorption rate 0.17 h(-1) (99%), absorption lag time 0.82 h, apparent nelfinavir total clearance 52 l h(-1) (49%), apparent nelfinavir volume of distribution 191 l, M8 elimination rate constant 1.76 h(-1) and nelfinavir to M8 0.39 h(-1) (59%) in *1/*1 patients and 0.20 h(-1) in *1/*2 or *2/*2 patients for CYP2C19*2. Nelfinavir C(mean) was positively correlated to glycaemia and triglyceride increases (P = 0.02 and P = 0.04, respectively).
Conclusions: The rate of metabolism of nelfinavir to M8 was reduced by 50% in patients with *1/*2 or *2/*2 genotype for CYP2C19 compared with those with *1/*1 genotype.
Figures

References
-
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigations. N Engl J Med. 1998;338:853–60. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ, Feinberg JE, Balfour HH, Deyton LR, Chodakewitz JA, Fischl MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–33. - PubMed
-
- Viracept package insert, 2001; Agouron Pharmaceuticals, La Jolla, CA, USA
-
- Lillibridge JH, Lee CA, Pithavala YK. XII Annual Meeting and Exposition of the American Association of Pharmaceutical Scientists. Arlington, VA: American Association of Pharmaceutical Scientists; 1998. [27 September 2007]. The role of polymorphic CYP2C19 in the metabolism of nelfinavir mesylate. Abstract 3035. Available at http://www.aapspharmsci.org/abstracts/AM_1998/1156.html.

