Pharmacokinetics of recombinant human growth hormone administered by cool.click 2, a new needle-free device, compared with subcutaneous administration using a conventional syringe and needle
- PMID: 17922895
- PMCID: PMC2093927
- DOI: 10.1186/1472-6904-7-10
Pharmacokinetics of recombinant human growth hormone administered by cool.click 2, a new needle-free device, compared with subcutaneous administration using a conventional syringe and needle
Abstract
Background: Growth hormone (GH) is used to treat growth hormone deficiency (GHD, adult and paediatric), short bowel syndrome in patients on a specialized diet, HIV-associated wasting and, in children, growth failure due to a number of disorders including Turner's syndrome and chronic renal failure, and in children born small for gestational age. Different brands and generic forms of recombinant human growth hormone (r-hGH) are approved for varying indications in different countries. New ways of administering GH are required because the use of a needle and syringe or a device where a patient still has to insert the needle manually into the skin on a daily basis can lead to low adherence and sub-optimal treatment outcomes. The objective of this study was to assess the relative bioavailability of r-hGH (Saizen, Merck Serono) administered by a new needle-free device, cool.click 2, and a standard needle and syringe.
Methods: The study was performed with 38 healthy volunteers who underwent pituitary somatotrope cell down-regulation using somatostatin, according to a randomized, two-period, two-sequence crossover design. Following subcutaneous administration of r-hGH using cool.click 2 or needle and syringe, pharmacokinetic parameters were analysed by non-compartmental methods. Bioequivalence was assessed based on log-transformed AUC and C(max) values.
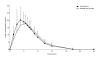
Results: The 90% confidence intervals for test/reference mean ratio of the plasma pharmacokinetic variables Cmax and AUC(0-inf) were 103.7-118.3 and 97.1-110.0, respectively, which is within the accepted bioequivalence range of 80-125%. r-hGH administered by cool.click 2 is, therefore, bioequivalent to administration by needle and syringe with respect to the rate and extent of GH exposure. Treatment using cool.click 2 was found to be well tolerated. With cool.click 2 the tmax was less (3.0 hours) than for needle and syringe delivery (4.5 hours), p = 0.002 (Friedman test), although this is unlikely to have any clinical implications.
Conclusion: These results demonstrate that cool.click 2 delivers subcutaneous r-hGH exposure that is bioequivalent to the conventional mode of injection. The new device has the additional advantage of being needle-free, and should help to increase patient adherence and achieve good therapeutic outcomes from r-hGH treatment.
Figures
Similar articles
-
Pharmacokinetics and pharmacodynamics of a new formulation of recombinant human growth hormone administered by ZomaJet 2 Vision, a new needle-free device, compared to subcutaneous administration using a conventional syringe.J Clin Pharmacol. 2002 Nov;42(11):1262-8. doi: 10.1177/009127002762491361. J Clin Pharmacol. 2002. PMID: 12412826 Clinical Trial.
-
Relative Bioavailability of a Single 4-mg Dose of Somatropin Administered by Subcutaneous Injection or by Needle-free Device and Coadministered With the Growth Hormone Inhibitor Octreotide Acetate in Healthy Adult Subjects.Clin Ther. 2018 May;40(5):741-751. doi: 10.1016/j.clinthera.2018.03.017. Epub 2018 Apr 24. Clin Ther. 2018. PMID: 29699852 Clinical Trial.
-
Are needle-free injections a useful alternative for growth hormone therapy in children? Safety and pharmacokinetics of growth hormone delivered by a new needle-free injection device compared to a fine gauge needle.J Pediatr Endocrinol Metab. 2003 Mar;16(3):383-92. doi: 10.1515/jpem.2003.16.3.383. J Pediatr Endocrinol Metab. 2003. PMID: 12705363 Clinical Trial.
-
Growth hormone therapy in children and adolescents: pharmacokinetic/pharmacodynamic considerations and emerging indications.Expert Opin Drug Metab Toxicol. 2008 Dec;4(12):1569-80. doi: 10.1517/17425250802465347. Expert Opin Drug Metab Toxicol. 2008. PMID: 19040332 Review.
-
Update from the GHMonitorSM observational registry in children treated with recombinant human growth hormone (Saizen).Pediatr Endocrinol Rev. 2009 Jan;6 Suppl 2:278-82. Pediatr Endocrinol Rev. 2009. PMID: 19337182 Review.
Cited by
-
Needle-Free and Needle-Based Growth Hormone Therapy in Children: A Pooled Analysis of Three Long-Term Observational Studies.Horm Res Paediatr. 2018;90(6):393-406. doi: 10.1159/000496614. Epub 2019 Mar 5. Horm Res Paediatr. 2018. PMID: 30836359 Free PMC article.
-
Formulations for children: problems and solutions.Br J Clin Pharmacol. 2015 Mar;79(3):405-18. doi: 10.1111/bcp.12268. Br J Clin Pharmacol. 2015. PMID: 25855822 Free PMC article. Review.
-
Needle-free injection of 5-aminolevulinic acid in photodynamic therapy for the treatment of condylomata acuminata.Exp Ther Med. 2013 Jul;6(1):236-240. doi: 10.3892/etm.2013.1092. Epub 2013 Apr 29. Exp Ther Med. 2013. PMID: 23935753 Free PMC article.
-
Gene therapy by electroporation for the treatment of chronic renal failure in companion animals.BMC Biotechnol. 2009 Jan 16;9:4. doi: 10.1186/1472-6750-9-4. BMC Biotechnol. 2009. PMID: 19149896 Free PMC article.
-
Developments in administration of growth hormone treatment: focus on Norditropin® Flexpro®.Patient Prefer Adherence. 2011 Mar 10;5:117-24. doi: 10.2147/PPA.S10985. Patient Prefer Adherence. 2011. PMID: 21448295 Free PMC article.
References
-
- McGauley G. The psychological consequences and quality of life in adults with growth hormone deficiency. Growth Horm IGF Res. 2000;10 Suppl B:S63–S68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources