17beta-Estradiol utilizes the estrogen receptor to regulate CD16 expression in monocytes
- PMID: 17923257
- PMCID: PMC2128779
- DOI: 10.1016/j.mce.2007.08.014
17beta-Estradiol utilizes the estrogen receptor to regulate CD16 expression in monocytes
Abstract
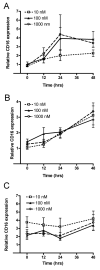
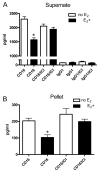
Estrogen can significantly influence CD16 expression and alter monocytic cytokine release upon CD16 receptor activation. However, the function of the estrogen receptor (ER) alpha and beta in this response is unclear. To test whether estrogen binds ERalpha and/or ERbeta to affect CD16 expression, monocytic cells were treated with and without physiological levels of 17beta-estradiol and various doses of the ERalpha and ERbeta antagonist fulvestrant followed by measurement of CD16 transcript levels. To determine how estrogen induced changes in TNF-alpha and IL-1beta release due to CD16 activation we quantitated the amount of cytokines after treatment with estrogen, fulvestrant and antibodies that specifically bind and activate the CD16 receptor. Interaction of ERalpha and the CD16 promoter was then determined by chromatin immunoprecipitation. Furthermore, specific promoter elements utilized by estrogen to control CD16 expression were mutated and expression from a luciferase reporter quantitated after transfection. Using the luciferase reporter construct containing a wild type CD16 promoter, the role of ERalpha and ERbeta in the estrogen response was tested by treating transfected monocytes with an ERalpha specific agonist or an ERbeta specific agonist and measuring expression. Our results show that CD16 transcript levels significantly decreased in monocytic cells due to estrogen and that the observed decrease in message was blocked by the antagonist fulvestrant. Estrogen reduced CD16 expression and decreased TNF-alpha and IL-1beta release upon CD16 activation but the administration of fulvestrant blocked this decrease. ERalpha was found to interact with a region 5' of the CD16 gene in the presence of estrogen, and site-directed mutational analysis of this region indicated the necessity for an estrogen response element in modulating estrogen effects on CD16 expression. Moreover, both an ERalpha and an ERbeta agonist reduced expression of the CD16 reporter construct suggesting both receptors can play a role in CD16 regulation. In conclusion, CD16 expression can be altered by the activity of ERalpha or ERbeta and our results also show that ERalpha can associate with a region within the CD16 promoter that is important in production of transcript.
Figures


Similar articles
-
Estrogen modulates in vitro T cell responses in a concentration- and receptor-dependent manner: effects on intracellular molecular targets and antioxidant enzymes.Mol Immunol. 2013 Dec;56(4):328-39. doi: 10.1016/j.molimm.2013.05.226. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911387
-
Phenytoin is an estrogen receptor α-selective modulator that interacts with helix 12.Reprod Sci. 2015 Feb;22(2):146-55. doi: 10.1177/1933719114549853. Epub 2014 Sep 25. Reprod Sci. 2015. PMID: 25258361
-
Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters.Mol Endocrinol. 2006 Mar;20(3):534-43. doi: 10.1210/me.2005-0140. Epub 2005 Nov 17. Mol Endocrinol. 2006. PMID: 16293641
-
Estrogen receptor-alpha gene expression in the cortex: sex differences during development and in adulthood.Horm Behav. 2011 Mar;59(3):353-7. doi: 10.1016/j.yhbeh.2010.08.004. Epub 2010 Aug 14. Horm Behav. 2011. PMID: 20713055 Free PMC article. Review.
-
Dynamic regulation of estrogen receptor-alpha gene expression in the brain: a role for promoter methylation?Front Neuroendocrinol. 2008 Jun;29(3):375-85. doi: 10.1016/j.yfrne.2008.03.002. Epub 2008 Mar 13. Front Neuroendocrinol. 2008. PMID: 18439661 Free PMC article. Review.
Cited by
-
Racial and Gender-Based Differences in COVID-19.Front Public Health. 2020 Jul 28;8:418. doi: 10.3389/fpubh.2020.00418. eCollection 2020. Front Public Health. 2020. PMID: 32850607 Free PMC article. Review.
-
Estrogen Receptor Functions and Pathways at the Vascular Immune Interface.Int J Mol Sci. 2021 Apr 20;22(8):4254. doi: 10.3390/ijms22084254. Int J Mol Sci. 2021. PMID: 33923905 Free PMC article. Review.
-
Decoding sex differences in human immunity through systems immunology.Oxf Open Immunol. 2025 Jul 4;6(1):iqaf006. doi: 10.1093/oxfimm/iqaf006. eCollection 2025. Oxf Open Immunol. 2025. PMID: 40692743 Free PMC article. Review.
-
The role of estradiol in the immune response against COVID-19.Hormones (Athens). 2021 Dec;20(4):657-667. doi: 10.1007/s42000-021-00300-7. Epub 2021 Jun 17. Hormones (Athens). 2021. PMID: 34142358 Free PMC article. Review.
-
Aromatase Inhibitors-Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects.Int J Mol Sci. 2020 Aug 6;21(16):5625. doi: 10.3390/ijms21165625. Int J Mol Sci. 2020. PMID: 32781535 Free PMC article. Review.
References
-
- Abrahams VM, Cambridge G, Lydyard PM, Edwards JC. Induction of tumor necrosis factor alpha production by adhered human monocytes: a key role for Fcgamma receptor type IIIa in rheumatoid arthritis. Arthritis Rheum. 2000;43:608–616. - PubMed
-
- Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. - PubMed
-
- Baeten D, Boots AM, Steenbakkers PG, Elewaut D, Bos E, Verheijden GF, Berheijden G, Miltenburg AM, Rijnders AW, Veys EM, De KF. Human cartilage gp-39+,CD16+ monocytes in peripheral blood and synovium: correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 2000;43:1233–1243. - PubMed
-
- Beaven MA, Metzger H. Signal transduction by Fc receptors: the Fc epsilon RI case. Immunol Today. 1993;14:222–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

