Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin
- PMID: 17923505
- PMCID: PMC2118486
- DOI: 10.1084/jem.20071262
Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin
Abstract
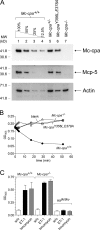
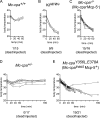
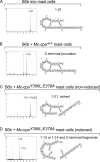
Mast cells are protective against snake venom sarafotoxins that belong to the endothelin (ET) peptide family. The molecular mechanism underlying this recently recognized innate defense pathway is unknown, but secretory granule proteases have been invoked. To specifically disrupt a single protease function without affecting expression of other proteases, we have generated a mouse mutant selectively lacking mast cell carboxypeptidase A (Mc-cpa) activity. Using this mutant, we have now identified Mc-cpa as the essential protective mast cell enzyme. Mass spectrometry of peptide substrates after cleavage by normal or mutant mast cells showed that removal of a single amino acid, the C-terminal tryptophan, from ET and sarafotoxin by Mc-cpa is the principle molecular mechanism underlying this very rapid mast cell response. Mast cell proteases can also cleave ET and sarafotoxin internally, but such "nicking" is not protective because intramolecular disulfide bridges maintain peptide function. We conclude that mast cells attack ET and sarafotoxin exactly at the structure required for toxicity, and hence sarafotoxins could not "evade" Mc-cpa's substrate specificity without loss of toxicity.
Figures




Similar articles
-
Novel insights into the biological function of mast cell carboxypeptidase A.Trends Immunol. 2009 Aug;30(8):401-8. doi: 10.1016/j.it.2009.04.008. Epub 2009 Jul 28. Trends Immunol. 2009. PMID: 19643669 Review.
-
Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice.J Clin Invest. 2011 Oct;121(10):4180-91. doi: 10.1172/JCI46139. Epub 2011 Sep 19. J Clin Invest. 2011. PMID: 21926462 Free PMC article.
-
Loss of histochemical identity in mast cells lacking carboxypeptidase A.Mol Cell Biol. 2005 Jul;25(14):6199-210. doi: 10.1128/MCB.25.14.6199-6210.2005. Mol Cell Biol. 2005. PMID: 15988029 Free PMC article.
-
Cloning of cDNAs that encode human mast cell carboxypeptidase A, and comparison of the protein with mouse mast cell carboxypeptidase A and rat pancreatic carboxypeptidases.Proc Natl Acad Sci U S A. 1989 Dec;86(23):9480-4. doi: 10.1073/pnas.86.23.9480. Proc Natl Acad Sci U S A. 1989. PMID: 2594780 Free PMC article.
-
Endothelins and sarafotoxins: peptides of similar structure and different function.Acta Medica (Hradec Kralove). 2004;47(3):157-62. Acta Medica (Hradec Kralove). 2004. PMID: 15568731 Review.
Cited by
-
Approaches for analyzing the roles of mast cells and their proteases in vivo.Adv Immunol. 2015;126:45-127. doi: 10.1016/bs.ai.2014.11.002. Epub 2015 Feb 7. Adv Immunol. 2015. PMID: 25727288 Free PMC article. Review.
-
Mast cell peptidases: chameleons of innate immunity and host defense.Am J Respir Cell Mol Biol. 2010 Mar;42(3):257-67. doi: 10.1165/rcmb.2009-0324RT. Epub 2009 Nov 20. Am J Respir Cell Mol Biol. 2010. PMID: 19933375 Free PMC article. Review.
-
Roles of IgE and Histamine in Mast Cell Maturation.Cells. 2021 Aug 23;10(8):2170. doi: 10.3390/cells10082170. Cells. 2021. PMID: 34440939 Free PMC article. Review.
-
The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5.J Immunol. 2010 Dec 15;185(12):7681-90. doi: 10.4049/jimmunol.1002803. Epub 2010 Nov 12. J Immunol. 2010. PMID: 21076070 Free PMC article.
-
Carboxypeptidase A3-A Key Component of the Protease Phenotype of Mast Cells.Cells. 2022 Feb 6;11(3):570. doi: 10.3390/cells11030570. Cells. 2022. PMID: 35159379 Free PMC article. Review.
References
-
- Echtenacher, B., D.N. Mannel, and L. Hultner. 1996. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 381:75–77. - PubMed
-
- Malaviya, R., T. Ikeda, E. Ross, and S.N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 381:77–80. - PubMed
-
- Wort, S.J., and T.W. Evans. 1999. The role of the endothelium in modulating vascular control in sepsis and related conditions. Br. Med. Bull. 55:30–48. - PubMed
-
- Wanecek, M., E. Weitzberg, A. Rudehill, and A. Oldner. 2000. The endothelin system in septic and endotoxin shock. Eur. J. Pharmacol. 407:1–15. - PubMed
-
- Magder, S., and P. Cernacek. 2003. Role of endothelins in septic, cardiogenic, and hemorrhagic shock. Can. J. Physiol. Pharmacol. 81:635–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

