Macrophage-mediated but gamma interferon-independent innate immune responses control the primary wave of Plasmodium yoelii parasitemia
- PMID: 17923512
- PMCID: PMC2168355
- DOI: 10.1128/IAI.01005-07
Macrophage-mediated but gamma interferon-independent innate immune responses control the primary wave of Plasmodium yoelii parasitemia
Abstract
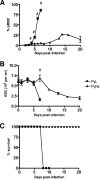
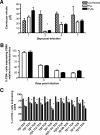
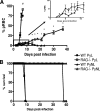
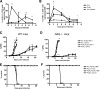
In most models of blood-stage malaria infection, proinflammatory immune responses are required for control of infection and elimination of parasites. We hypothesized therefore that the fulminant infections caused in mice by the lethal strain of Plasmodium yoelii (17XL) might be due to failure to activate a sufficient inflammatory response. Here we have compared the adaptive CD4+ T-cell and innate immune response to P. yoelii 17XL with that induced by the self-resolving, nonlethal strain of P. yoelii, 17X(NL). During the first 7 to 9 days of infection, splenic effector CD4+ T-cell responses were similar in mice with lethal and nonlethal infections with similar levels of activation in vivo and equivalent proliferation in vitro following mitogenic stimulation. Nonspecific T-cell hyporesponsiveness was observed at similar levels during both infections and was due, in part, to suppression mediated by CD11b+ cells. Importantly, however, RAG-/- mice were able to control the initial growth phase of nonlethal P. yoelii infection as effectively as wild-type mice, indicating that T cells and/or B cells play little, if any, role in control of the primary peak of parasitemia. Somewhat unexpectedly, we could find no clear role for either NK cells or gamma interferon (IFN-gamma) in controlling primary P. yoelii infection. In contrast, depletion of monocytes/macrophages exacerbated parasite growth and anemia during both lethal and nonlethal acute P. yoelii infections, indicating that there is an IFN-gamma-, NK cell-, and T-cell-independent pathway for induction of effector macrophages during acute malaria infection.
Figures


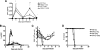

References
-
- Akimitsu, N., H. S. Kim, H. Hamamoto, K. Kamura, N. Fukuma, N. Arimitsu, K. Ono, Y. Wataya, M. Torii, and K. Sekimizu. 2004. Duffy antigen is important for the lethal effect of the lethal strain of Plasmodium yoelii 17XL. Parasitol. Res. 93:499-503. - PubMed
-
- Amante, F. H., and M. F. Good. 1997. Prolonged Th1-like response generated by a Plasmodium yoelii-specific T cell clone allows complete clearance of infection in reconstituted mice. Parasite Immunol. 19:111-126. - PubMed
-
- Brake, D. A., W. P. Weidanz, and C. A. Long. 1986. Antigen-specific, interleukin 2-propagated T lymphocytes confer resistance to a murine malarial parasite, Plasmodium chabaudi adami. J. Immunol. 137:347-352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

