High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica
- PMID: 17923515
- PMCID: PMC2168356
- DOI: 10.1128/IAI.00559-07
High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica
Abstract
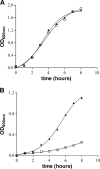
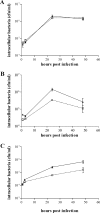
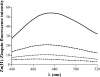
To investigate the relevance of zinc in host-pathogen interactions, we have constructed Salmonella enterica mutant strains in which the znuA gene, which encodes the periplasmic component of the ZnuABC high-affinity Zn2+ transporter, was deleted. This mutation does not alter the ability of Salmonella to grow in rich media but drastically reduces its ability to multiply in media deprived of zinc. In agreement with this phenotype, ZnuA accumulates only in bacteria cultivated in environments poor in zinc. In spite of the nearly millimolar intracellular concentration of zinc, we have found that znuA is highly expressed in intracellular salmonellae recovered either from cultivated cells or from the spleens of infected mice. We have also observed that znuA mutants are impaired in their ability to grow in Caco-2 epithelial cells and that bacteria starved for zinc display decreased ability to multiply in phagocytes. A dramatic reduction in the pathogenicity of the znuA mutants was observed in Salmonella-susceptible (BALB/c) or Salmonella-resistant (DBA-2) mice infected intraperitoneally or orally. This study shows that the amount of free metals available for bacterial growth within the infected animal is limited, despite the apparent elevated concentration of free metals within cells and in plasma and suggests that Salmonella exploits the ZnuABC zinc transporter to maximize zinc availability in such conditions. These results shed new light on the complex functions of zinc in vertebrate and bacterial physiology and pave the way for a better comprehension of pathogenic mechanisms in Salmonella infections.
Figures




References
-
- Andreini, C., L. Banci, I. Bertini, and A. Rosato. 2006. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 5:196-201. - PubMed
-
- Berducci, G., A. P. Mazzetti, G. Rotilio, and A. Battistoni. 2004. Periplasmic competition for zinc uptake between the metallochaperone ZnuA and Cu,Zn superoxide dismutase. FEBS Lett. 569:289-292. - PubMed
-
- Blencowe, K., and P. Morby. 2003. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 27:291-311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

