Defective Ca2+ channel clustering in axon terminals disturbs excitability in motoneurons in spinal muscular atrophy
- PMID: 17923533
- PMCID: PMC2064743
- DOI: 10.1083/jcb.200703187
Defective Ca2+ channel clustering in axon terminals disturbs excitability in motoneurons in spinal muscular atrophy
Abstract
Proximal spinal muscular atrophy (SMA) is a motoneuron disease for which there is currently no effective treatment. In animal models of SMA, spinal motoneurons exhibit reduced axon elongation and growth cone size. These defects correlate with reduced beta-actin messenger RNA and protein levels in distal axons. We show that survival motoneuron gene (Smn)-deficient motoneurons exhibit severe defects in clustering Cav2.2 channels in axonal growth cones. These defects also correlate with a reduced frequency of local Ca2+ transients. In contrast, global spontaneous excitability measured in cell bodies and proximal axons is not reduced. Stimulation of Smn production from the transgenic SMN2 gene by cyclic adenosine monophosphate restores Cav2.2 accumulation and excitability. This may lead to the development of new therapies for SMA that are not focused on enhancing motoneuron survival but instead investigate restoration of growth cone excitability and function.
Figures

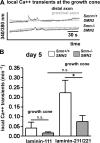
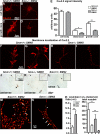

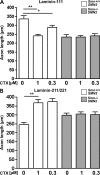


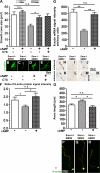
Similar articles
-
Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons.J Cell Biol. 2003 Nov 24;163(4):801-12. doi: 10.1083/jcb.200304128. Epub 2003 Nov 17. J Cell Biol. 2003. PMID: 14623865 Free PMC article.
-
Distinct and overlapping alterations in motor and sensory neurons in a mouse model of spinal muscular atrophy.Hum Mol Genet. 2006 Feb 1;15(3):511-8. doi: 10.1093/hmg/ddi467. Epub 2006 Jan 5. Hum Mol Genet. 2006. PMID: 16396995
-
Role of Na(v)1.9 in activity-dependent axon growth in motoneurons.Hum Mol Genet. 2012 Aug 15;21(16):3655-67. doi: 10.1093/hmg/dds195. Epub 2012 May 28. Hum Mol Genet. 2012. PMID: 22641814
-
Fishing for a mechanism: using zebrafish to understand spinal muscular atrophy.J Child Neurol. 2007 Aug;22(8):995-1003. doi: 10.1177/0883073807305671. J Child Neurol. 2007. PMID: 17761655 Review.
-
Pathogenesis of proximal autosomal recessive spinal muscular atrophy.Acta Neuropathol. 2008 Sep;116(3):223-34. doi: 10.1007/s00401-008-0411-1. Epub 2008 Jul 16. Acta Neuropathol. 2008. PMID: 18629520 Review.
Cited by
-
Calpain system is altered in survival motor neuron-reduced cells from in vitro and in vivo spinal muscular atrophy models.Cell Death Dis. 2020 Jun 25;11(6):487. doi: 10.1038/s41419-020-2688-5. Cell Death Dis. 2020. PMID: 32587237 Free PMC article.
-
Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy.Science. 2008 Apr 25;320(5875):524-7. doi: 10.1126/science.1155085. Science. 2008. PMID: 18440926 Free PMC article.
-
Stem cells: Tailor-made diseased neurons.Nature. 2009 Jan 15;457(7227):269-70. doi: 10.1038/457269a. Nature. 2009. PMID: 19148087 No abstract available.
-
Loss of synaptic Munc13-1 underlies neurotransmission abnormalities in spinal muscular atrophy.Cell Mol Life Sci. 2025 Aug 29;82(1):325. doi: 10.1007/s00018-025-05859-7. Cell Mol Life Sci. 2025. PMID: 40879740 Free PMC article.
-
Cell-autonomous axon growth of young motoneurons is triggered by a voltage-gated sodium channel.Channels (Austin). 2013 Jan 1;7(1):51-6. doi: 10.4161/chan.23153. Epub 2012 Dec 13. Channels (Austin). 2013. PMID: 23238424 Free PMC article.
References
-
- Boillee, S., V.C. Vande, and D.W. Cleveland. 2006. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 52:39–59. - PubMed
-
- Bruijn, L.I., T.M.Miller, and D.W.Cleveland. 2004. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 27:723–49. - PubMed
-
- Chan, Y.B., I. Miguel-Aliaga, C. Franks, N. Thomas, B. Trulzsch, D.B. Sattelle, K.E. Davies, and H.M. van den Heuvel. 2003. Neuromuscular defects in a Drosophila survival motor neuron gene mutant. Hum. Mol. Genet. 12:1367–1376. - PubMed
-
- Cifuentes-Diaz, C., S. Nicole, M.E. Velasco, C. Borra-Cebrian, C. Panozzo, T. Frugier, G. Millet, N. Roblot, V. Joshi, and J. Melki. 2002. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Hum. Mol. Genet. 11:1439–1447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

