Renal defects associated with improper polarization of the CRB and DLG polarity complexes in MALS-3 knockout mice
- PMID: 17923534
- PMCID: PMC2064744
- DOI: 10.1083/jcb.200702054
Renal defects associated with improper polarization of the CRB and DLG polarity complexes in MALS-3 knockout mice
Abstract
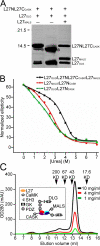
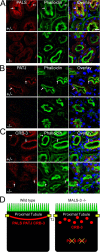
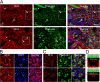
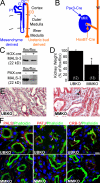
Kidney development and physiology require polarization of epithelia that line renal tubules. Genetic studies show that polarization of invertebrate epithelia requires the crumbs, partition-defective-3, and discs large complexes. These evolutionarily conserved protein complexes occur in mammalian kidney; however, their role in renal development remains poorly defined. Here, we find that mice lacking the small PDZ protein mammalian LIN-7c (MALS-3) have hypomorphic, cystic, and fibrotic kidneys. Proteomic analysis defines MALS-3 as the only known core component of both the crumbs and discs large cell polarity complexes. MALS-3 mediates stable assembly of the crumbs tight junction complex and the discs large basolateral complex, and these complexes are disrupted in renal epithelia from MALS-3 knockout mice. Interestingly, MALS-3 controls apico-basal polarity preferentially in epithelia derived from metanephric mesenchyme, and defects in kidney architecture owe solely to MALS expression in these epithelia. These studies demonstrate that defects in epithelial cell polarization can cause cystic and fibrotic renal disease.
Figures





References
-
- Bachmann, A., M. Schneider, E. Theilenberg, F. Grawe, and E. Knust. 2001. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 414:638–643. - PubMed
-
- Bachmann, A., M. Timmer, J. Sierralta, G. Pietrini, E.D. Gundelfinger, E. Knust, and U. Thomas. 2004. Cell type-specific recruitment of Drosophila Lin-7 to distinct MAGUK-based protein complexes defines novel roles for Sdt and Dlg-S97. J. Cell Sci. 117:1899–1909. - PubMed
-
- Betschinger, J., K. Mechtler, and J.A. Knoblich. 2003. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 422:326–330. - PubMed
-
- Bhat, M.A., S. Izaddoost, Y. Lu, K.O. Cho, K.W. Choi, and H.J. Bellen. 1999. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 96:833–845. - PubMed
-
- Bilder, D., and N. Perrimon. 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 403:676–680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

