Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review
- PMID: 17923590
- PMCID: PMC2135553
- DOI: 10.1001/archinte.167.18.1922
Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review
Erratum in
- Arch Intern Med. 2007 Dec 10-22;167(22):2452
Abstract
Background: More than 180 different types of therapy have been used in the treatment and management of painful bladder syndrome/interstitial cystitis (PBS/IC), yet evidence from clinical trials remains inconclusive. This study aimed to evaluate the efficacy of pharmacologic approaches to PBS/IC, to quantify the effect size from randomized controlled trials, and to begin to inform a clinical consensus of treatment efficacy for PBS/IC.
Methods: We identified randomized controlled trials for the pharmacologic treatment of patients with PBS/IC diagnosed on the basis of National Institute of Diabetes and Digestive and Kidney Diseases or operational criteria. Study limitations include considerable patient heterogeneity as well as variability in the definition of symptoms and in outcome assessment.
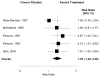
Results: We included a total of 1470 adult patients from 21 randomized controlled trials. Only trials for pentosan polysulfate sodium had sufficient numbers to allow a pooled analysis of effect. According to a random-effects model, the pooled estimate of the effect of pentosan polysulfate therapy suggested benefit, with a relative risk of 1.78 for patient-reported improvement in symptoms (95% confidence interval, 1.34-2.35). This result was not heterogeneous (P = .47) and was without evidence of publication bias (P = .18). Current evidence also suggests the efficacy of dimethyl sulfoxide and amitryptyline therapy. Hydroxyzine, intravesical bacille Calmette-Guérin, and resiniferatoxin therapy failed to demonstrate efficacy, but evidence was inconclusive owing to methodological limitations.
Conclusions: Pentosan polysulfate may be modestly beneficial for symptoms of PBS/IC. There is insufficient evidence for other pharmacologic treatments. A consensus on standardized outcome measures is urgently needed.
Figures
References
-
- Vaughan E, Wilt T, Hanno P, Curhan GC. National Institutes of Diabetes and Digestive and Kidney Diseases/National Institutes of Health. Bethesda, MD: 2003. [Accessed February 26, 2007]. Interstitial Cystitis Epidemiology Task Force Meeting Executive Committee Summary. Available at http://www.niddk.nih.gov/fund/reports/ic/executive_summary.htm.
-
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. - PubMed
-
- Hunner GL. A rare type of bladder ulcer in women; report of cases. Boston Med Surg Journal. 1915;172:660–664.
-
- Messing EM, Stamey TA. Interstitial cystitis: early diagnosis, pathology, and treatment. Urology. 1978;12:381–92. - PubMed
-
- Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health, Bethesda, Maryland, August 28–29, 1987. J Urol. 1988;140:203–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical