Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial
- PMID: 17923654
- PMCID: PMC1995143
- DOI: 10.1503/cmaj.061339
Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial
Abstract
Background: Physical function and exercise capacity decline with age and are a major source of disability in older people. Recent evidence suggests a potential role for the renin-angiotensin system in modulating muscle function. We sought to examine the effect of the angiotensin-converting-enzyme (ACE) inhibitor perindopril on physical function in elderly people with functional impairment who had no heart failure or left ventricular systolic dysfunction.
Methods: In this double-blind randomized controlled trial, participants aged 65 years and older who had problems with mobility or functional impairment were randomly assigned to receive either perindopril or placebo for 20 weeks. The primary outcome was the change in the 6-minute walking distance over the 20 weeks. Secondary outcomes were changes in muscle function, daily activity levels, self-reported function and health-related quality of life.
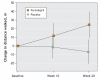
Results: A total of 130 participants were enrolled in the study (mean age 78.7, standard deviation 7.7 years); 95 completed the trial. At 20 weeks, the mean 6-minute walking distance was significantly improved in the perindopril group relative to the placebo group (mean between-group difference 31.4 m, 95% confidence interval [CI] 10.8 to 51.9 m; p = 0.003). There was a significant impact on health-related quality of life: although the mean score for part 1 of the EQ-5D questionnaire deteriorated over time in the placebo group, quality of life was maintained in the perindopril group, for a between-group difference of 0.09 (p = 0.046). There were no significant differences between the 2 groups in the other outcomes.
Interpretation: Use of the ACE inhibitor perindopril improved exercise capacity in functionally impaired elderly people who had no heart failure and maintained health-related quality of life. The degree of improvement was equivalent to that reported after 6 months of exercise training. (International Standard Randomised Controlled Trial Register no. ISRCTN67679521).
Figures
Comment in
-
Is there a new role for angiotensin-converting-enzyme inhibitors in elderly patients?CMAJ. 2007 Oct 9;177(8):891-2. doi: 10.1503/cmaj.071062. CMAJ. 2007. PMID: 17923657 Free PMC article. No abstract available.
References
-
- Henriksen EJ, Jacob S. Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. J Cell Physiol 2003;196:171-9. - PubMed
-
- Onder G, Penninx BW, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet 2002;359:926-30. - PubMed
-
- Montgomery H, Clarkson P, Barnard M, et al. Angiotensin-converting-enzyme gene insertion/deletion polymorphism and response to physical training. Lancet 1999;353:541-5. - PubMed
-
- Remme WJ, Swedberg K. Comprehensive guidelines for the diagnosis and treatment of chronic heart failure. Task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Eur J Heart Fail 2002;4:11-22. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous