Efficient in vivo targeting of epidermal stem cells by early gestational intraamniotic injection of lentiviral vector driven by the keratin 5 promoter
- PMID: 17923841
- PMCID: PMC3147185
- DOI: 10.1038/sj.mt.6300332
Efficient in vivo targeting of epidermal stem cells by early gestational intraamniotic injection of lentiviral vector driven by the keratin 5 promoter
Abstract
At the present time, no efficient in vivo method for gene transfer to skin stem cells exists. In this study, we hypothesized that early in gestation, specific epidermal stem cell populations may be accessible for gene transfer. To test this hypothesis, we injected lentiviral vectors encoding the green fluorescence protein marker gene driven by either the cytomegalovirus promoter or the keratin 5 (K5) promoter into the murine amniotic space at early developmental stages between embryonic days 8 and 12. This resulted in sustained green fluorescent protein (GFP) expression in both basal epidermal stem cells and bulge cells in the hair follicles of the skin. Transduction of stem cell populations was dependent on the developmental stage, and confirmed by the prolonged duration of GFP expression in all skin elements into adulthood. In addition, transduced stem cell populations responded to regenerative signals after wounding and actively participated in wound healing. Finally, we quantified the fraction of epidermal stem cells transduced, and the distribution of transduction related to the promoters utilized, confirming improved efficiency with the K5 promoter. This simple approach has possible biological applications in our study of gene functions in skin, and perhaps future clinical applications for treatment of skin based disorders.
Figures
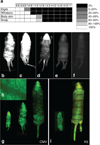
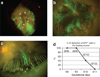

References
-
- Pulkkinen L, Ringpfeil F, Uitto J. Progress in heritable skin diseases: molecular bases and clinical implications. J Am Acad Dermatol. 2002;47:91–104. - PubMed
-
- Uitto J, Pulkkinen L. The genodermatoses: candidate diseases for gene therapy. Hum Gene Ther. 2000;11:2267–2275. - PubMed
-
- Hengge UR, Bardenheuer W. Gene therapy and the skin. Am J Med Genet C Semin Med Genet. 2004;131C:93–100. - PubMed
-
- Woodley DT, Keene DR, Atha T, Huang Y, Ram R, Kasahara N, et al. Intradermal injection of lentiviral vectors corrects regenerated human dystrophic epidermolysis bullosa skin tissue in vivo. Mol Ther. 2004;10:318–326. - PubMed
-
- Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397–1402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

