Tie-dyed1 and sucrose export defective1 act independently to promote carbohydrate export from maize leaves
- PMID: 17924136
- PMCID: PMC2249615
- DOI: 10.1007/s00425-007-0636-6
Tie-dyed1 and sucrose export defective1 act independently to promote carbohydrate export from maize leaves
Abstract
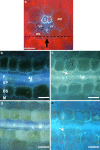
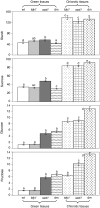
tie-dyed1 (tdy1) and sucrose export defective1 (sxd1) are recessive maize (Zea mays) mutants with nonclonal chlorotic leaf sectors that hyperaccumulate starch and soluble sugars. In addition, both mutants display similar growth-related defects such as reduced plant height and inflorescence development due to the retention of carbohydrates in leaves. As tdy1 and sxd1 are the only variegated leaf mutants known to accumulate carbohydrates in any plant, we investigated whether Tdy1 and Sxd1 function in the same pathway. Using aniline blue staining for callose and transmission electron microscopy to inspect plasmodesmatal ultrastructure, we determined that tdy1 does not have any physical blockage or alteration along the symplastic transport pathway as found in sxd1 mutants. To test whether the two genes function in the same genetic pathway, we constructed F(2) families segregating both mutations. Double mutant plants showed an additive interaction for growth related phenotypes and soluble sugar accumulation, and expressed the leaf variegation pattern of both single mutants indicating that Tdy1 and Sxd1 act in separate genetic pathways. Although sxd1 mutants lack tocopherols, we determined that tdy1 mutants have wild-type tocopherol levels, indicating that Tdy1 does not function in the same biochemical pathway as Sxd1. From these and other data we conclude that Tdy1 and Sxd1 function independently to promote carbon export from leaves. Our genetic and cytological studies implicate Tdy1 functioning in veins, and a model discussing possible functions of TDY1 is presented.
Figures




Similar articles
-
Tie-dyed2 functions with tie-dyed1 to promote carbohydrate export from maize leaves.Plant Physiol. 2008 Mar;146(3):1085-97. doi: 10.1104/pp.107.111476. Epub 2008 Jan 24. Plant Physiol. 2008. PMID: 18218972 Free PMC article.
-
tie-dyed1 Functions non-cell autonomously to control carbohydrate accumulation in maize leaves.Plant Physiol. 2007 Jun;144(2):867-78. doi: 10.1104/pp.107.098814. Epub 2007 Apr 13. Plant Physiol. 2007. PMID: 17434986 Free PMC article.
-
tie-dyed1 Regulates carbohydrate accumulation in maize leaves.Plant Physiol. 2006 Dec;142(4):1511-22. doi: 10.1104/pp.106.090381. Epub 2006 Oct 27. Plant Physiol. 2006. PMID: 17071639 Free PMC article.
-
Tocochromanol functions in plants: antioxidation and beyond.J Exp Bot. 2010 Jun;61(6):1549-66. doi: 10.1093/jxb/erq030. J Exp Bot. 2010. PMID: 20385544 Review.
-
Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security.J Exp Bot. 2014 Apr;65(7):1713-35. doi: 10.1093/jxb/ert416. Epub 2013 Dec 17. J Exp Bot. 2014. PMID: 24347463 Review.
Cited by
-
Callose deposition in the phloem plasmodesmata and inhibition of phloem transport in citrus leaves infected with "Candidatus Liberibacter asiaticus".Protoplasma. 2012 Jul;249(3):687-97. doi: 10.1007/s00709-011-0312-3. Epub 2011 Aug 28. Protoplasma. 2012. PMID: 21874517
-
Maize Brittle Stalk2-Like3, encoding a COBRA protein, functions in cell wall formation and carbohydrate partitioning.Plant Cell. 2021 Oct 11;33(10):3348-3366. doi: 10.1093/plcell/koab193. Plant Cell. 2021. PMID: 34323976 Free PMC article.
-
The molecular cloning and clarification of a photorespiratory mutant, oscdm1, using enhancer trapping.Front Genet. 2015 Jul 3;6:226. doi: 10.3389/fgene.2015.00226. eCollection 2015. Front Genet. 2015. PMID: 26191072 Free PMC article.
-
Tie-dyed2 encodes a callose synthase that functions in vein development and affects symplastic trafficking within the phloem of maize leaves.Plant Physiol. 2012 Nov;160(3):1540-50. doi: 10.1104/pp.112.202473. Epub 2012 Aug 29. Plant Physiol. 2012. PMID: 22932757 Free PMC article.
-
Sucrose Transporter ZmSut1 Expression and Localization Uncover New Insights into Sucrose Phloem Loading.Plant Physiol. 2016 Nov;172(3):1876-1898. doi: 10.1104/pp.16.00884. Epub 2016 Sep 12. Plant Physiol. 2016. PMID: 27621426 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '10589520', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10589520/'}]}
- Aoki N, Hirose T, Takahashi S, Ono K, Ishimaru K, Ohsugi R (1999) Molecular cloning and expression analysis of a gene for a sucrose transporter in maize (Zea mays L.). Plant Cell Physiol 40:1072–1078 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1104/pp.107.098814', 'is_inner': False, 'url': 'https://doi.org/10.1104/pp.107.098814'}, {'type': 'PMC', 'value': 'PMC1914200', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1914200/'}, {'type': 'PubMed', 'value': '17434986', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17434986/'}]}
- Baker RF, Braun DM (2007) tie-dyed1 functions non-cell autonomously to control carbohydrate accumulation in maize leaves. Plant Physiol 144:867–878 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/BF02524263', 'is_inner': False, 'url': 'https://doi.org/10.1007/bf02524263'}]}
- Botha CEJ, Cross RHM, van Bel AJE, Peter CI (2000) Phloem loading in the sucrose-export-defective (SXD-1) mutant maize is limited by callose deposition at plasmodesmata in bundle sheath–vascular parenchyma interface. Protoplasma 214:65–72
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1104/pp.106.090381', 'is_inner': False, 'url': 'https://doi.org/10.1104/pp.106.090381'}, {'type': 'PMC', 'value': 'PMC1676051', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1676051/'}, {'type': 'PubMed', 'value': '17071639', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17071639/'}]}
- Braun DM, Ma Y, Inada N, Muszynski MG, Baker RF (2006) tie-dyed1 regulates carbohydrate accumulation in maize leaves. Plant Physiol 142:1511–1522 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1104/pp.118.1.59', 'is_inner': False, 'url': 'https://doi.org/10.1104/pp.118.1.59'}, {'type': 'PMC', 'value': 'PMC34874', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC34874/'}, {'type': 'PubMed', 'value': '9733526', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9733526/'}]}
- Burkle L, Hibberd JM, Quick WP, Kuhn C, Hirner B, Frommer WB (1998) The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol 118:59–68 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

