A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy
- PMID: 17924338
- PMCID: PMC2265660
- DOI: 10.1086/521633
A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy
Abstract
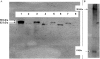
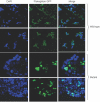
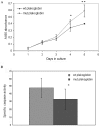
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited disorder associated with arrhythmias and sudden death. A recessive mutation in the gene encoding plakoglobin has been shown to cause Naxos disease, a cardiocutaneous syndrome characterized by ARVC and abnormalities of hair and skin. Here, we report, for the first time, a dominant mutation in the gene encoding plakoglobin in a German family with ARVC but no cutaneous abnormalities. The mutation (S39_K40insS) is predicted to insert an extra serine residue at position 39 in the N-terminus of plakoglobin. Analysis of a biopsy sample of the right ventricle from the proband showed markedly decreased localization of plakoglobin, desmoplakin, and connexin43 at intercalated discs in cardiac myocytes. A yeast-two-hybrid screen revealed that the mutant protein established novel interactions with histidine-rich calcium-binding protein and TGF beta induced apoptosis protein 2. Immunoblotting and confocal microscopy in human embryonic kidney 293 (HEK293) cell lines transfected to stably express either wild-type or mutant plakoglobin protein showed that the mutant protein was apparently ubiquitylated and was preferentially located in the cytoplasm, suggesting that the S39_K40insS mutation may increase plakoglobin turnover via proteasomal degradation. HEK293 cells expressing mutant plakoglobin also showed higher rates of proliferation and lower rates of apoptosis than did cells expressing the wild-type protein. Electron microscopy showed smaller and fewer desmosomes in cells expressing mutant plakoglobin. Taken together, these observations suggest that the S39_K40insS mutation affects the structure and distribution of mechanical and electrical cell junctions and could interfere with regulatory mechanisms mediated by Wnt-signaling pathways. These results implicate novel molecular mechanisms in the pathogenesis of ARVC.
Figures



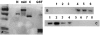
Similar articles
-
Arrhythmogenic right ventricular cardiomyopathy mutations alter shear response without changes in cell-cell adhesion.Cardiovasc Res. 2014 Nov 1;104(2):280-9. doi: 10.1093/cvr/cvu212. Epub 2014 Sep 24. Cardiovasc Res. 2014. PMID: 25253076 Free PMC article.
-
Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling.Mol Cell Biol. 2011 Mar;31(6):1134-44. doi: 10.1128/MCB.01025-10. Epub 2011 Jan 18. Mol Cell Biol. 2011. PMID: 21245375 Free PMC article.
-
Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease).Lancet. 2000 Jun 17;355(9221):2119-24. doi: 10.1016/S0140-6736(00)02379-5. Lancet. 2000. PMID: 10902626
-
Etiopathogenesis of arrhythmogenic right ventricular cardiomyopathy.J Hum Genet. 2005;50(8):375-381. doi: 10.1007/s10038-005-0273-5. Epub 2005 Aug 12. J Hum Genet. 2005. PMID: 16096717 Review.
-
Naxos disease: from the origin to today.Orphanet J Rare Dis. 2018 May 10;13(1):74. doi: 10.1186/s13023-018-0814-6. Orphanet J Rare Dis. 2018. PMID: 29747658 Free PMC article. Review.
Cited by
-
Established and Emerging Mechanisms in the Pathogenesis of Arrhythmogenic Cardiomyopathy: A Multifaceted Disease.Int J Mol Sci. 2020 Aug 31;21(17):6320. doi: 10.3390/ijms21176320. Int J Mol Sci. 2020. PMID: 32878278 Free PMC article. Review.
-
Intercalated discs: cellular adhesion and signaling in heart health and diseases.Heart Fail Rev. 2019 Jan;24(1):115-132. doi: 10.1007/s10741-018-9743-7. Heart Fail Rev. 2019. PMID: 30288656 Review.
-
A global perspective of arrhythmogenic right ventricular cardiomyopathy.Glob Cardiol Sci Pract. 2013 Nov 1;2012(2):81-92. doi: 10.5339/gcsp.2012.26. eCollection 2012. Glob Cardiol Sci Pract. 2013. PMID: 24688993 Free PMC article. Review.
-
Mechanisms of disease: molecular genetics of arrhythmogenic right ventricular dysplasia/cardiomyopathy.Nat Clin Pract Cardiovasc Med. 2008 May;5(5):258-67. doi: 10.1038/ncpcardio1182. Epub 2008 Apr 1. Nat Clin Pract Cardiovasc Med. 2008. PMID: 18382419 Free PMC article. Review.
-
Current insights into LMNA cardiomyopathies: Existing models and missing LINCs.Nucleus. 2017 Jan 2;8(1):17-33. doi: 10.1080/19491034.2016.1260798. Nucleus. 2017. PMID: 28125396 Free PMC article. Review.
References
Web Resource
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Naxos disease, PK2157del2, desmoplakin, plakophilin-2, desmoglein-2, desmocollin-2, HRC-BP, N-cadherin, Cx43, α-catenin, and RYR2)
References
-
- Thiene G, Nava A, Corrado D, Rossi L, Pennelli N (1988) Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 318:129–133 - PubMed
-
- McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ (2000) Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 355:2119–212410.1016/S0140-6736(00)02379-5 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

