Role of alphavbeta6 integrin in acute biliary fibrosis
- PMID: 17924447
- PMCID: PMC4144397
- DOI: 10.1002/hep.21849
Role of alphavbeta6 integrin in acute biliary fibrosis
Abstract
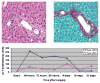
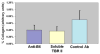
Acute biliary obstruction leads to periductal myofibroblasts and fibrosis, the origin of which is uncertain. Our study provides new information on this question in mice and humans. We show that bile duct obstruction induces a striking increase in cholangiocyte alphavbeta6 integrin and that expression of this integrin is directly linked to fibrogenesis through activation of transforming growth factor beta (TGF-beta). Administration of blocking antibody to alphavbeta6 significantly reduces the extent of acute fibrosis after bile duct ligation. Moreover, in beta6-null mice subjected to the injury, fibrosis is reduced by 50% relative to that seen in wild-type mice, whereas inflammation occurs to the same extent. The data indicate that alphavbeta6, rather than inflammation, is linked to fibrogenesis. It is known that alphavbeta6 binds latent TGF-beta and that binding results in release of active TGFbeta. Consistent with this, intracellular signaling from the TGFbeta receptor is increased after bile duct ligation in wild-type mice but not in beta6(-/-) mice, and a competitive inhibitor of the TGFbeta receptor type II blocks fibrosis to the same extent as antibody to alphavbeta6. In a survey of human liver disease, expression of alphavbeta6 is increased in acute, but not chronic, biliary injury and is localized to cholangiocyte-like cells.
Conclusion: Cholangiocytes respond to acute bile duct obstruction with markedly increased expression of alphavbeta6 integrin, which is closely linked to periductal fibrogenesis. The findings provide a rationale for the use of inhibitors of alphavbeta6 integrin or TGFbeta for down-regulating fibrosis in the setting of acute or ongoing biliary injury.
Conflict of interest statement
Potential conflict of interest: Dr. Violette and Dr. Weinreb own stock in Biogen Idec.
Figures









References
-
- Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol. 1998;15:259–269. - PubMed
-
- Alpini G, McGill JM, Larusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–1268. - PubMed
-
- Bissell DM. Chronic liver injury, TGF-beta, and cancer. Exp Mol Med. 2001;33:179–190. - PubMed
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
