Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons
- PMID: 17924530
- PMCID: PMC2408670
- DOI: 10.1002/hipo.20374
Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons
Abstract
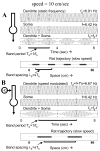
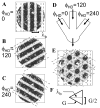
Intracellular recording and computational modelling suggest that interactions of subthreshold membrane potential oscillation frequency in different dendritic branches of entorhinal cortex stellate cells could underlie the functional coding of continuous dimensions of space and time. Among other things, these interactions could underlie properties of grid cell field spacing. The relationship between experimental data on membrane potential oscillation frequency (f) and grid cell field spacing (G) indicates a constant scaling factor H = fG. This constant scaling factor between temporal oscillation frequency and spatial periodicity provides a starting constraint that is used to derive the model of Burgess et al. (Hippocampus, 2007). This model provides a consistent quantitative link between single cell physiological properties and properties of spiking units in awake behaving animals. Further properties and predictions of this model about single cell and network physiological properties are analyzed. In particular, the model makes quantitative predictions about the change in membrane potential, single cell oscillation frequency, and network oscillation frequency associated with speed of movement, about the independence of single cell properties from network theta rhythm oscillations, and about the effect of variations in initial oscillatory phase on the pattern of grid cell firing fields. These same mechanisms of subthreshold oscillations may play a more general role in memory function, by providing a method for learning arbitrary time intervals in memory sequences.
(c) 2007 Wiley-Liss, Inc.
Figures










Similar articles
-
An oscillatory interference model of grid cell firing.Hippocampus. 2007;17(9):801-12. doi: 10.1002/hipo.20327. Hippocampus. 2007. PMID: 17598147 Free PMC article. Review.
-
Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing.Science. 2007 Mar 23;315(5819):1719-22. doi: 10.1126/science.1139207. Science. 2007. PMID: 17379810 Free PMC article.
-
How reduction of theta rhythm by medial septum inactivation may covary with disruption of entorhinal grid cell responses due to reduced cholinergic transmission.Front Neural Circuits. 2013 Oct 31;7:173. doi: 10.3389/fncir.2013.00173. eCollection 2013. Front Neural Circuits. 2013. PMID: 24198762 Free PMC article.
-
Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference.J Neurosci. 2011 Nov 9;31(45):16157-76. doi: 10.1523/JNEUROSCI.0712-11.2011. J Neurosci. 2011. PMID: 22072668 Free PMC article.
-
Linking cellular mechanisms to behavior: entorhinal persistent spiking and membrane potential oscillations may underlie path integration, grid cell firing, and episodic memory.Neural Plast. 2008;2008:658323. doi: 10.1155/2008/658323. Neural Plast. 2008. PMID: 18670635 Free PMC article. Review.
Cited by
-
In vivo cholinergic modulation of the cellular properties of medial entorhinal cortex neurons.J Physiol. 2013 May 15;591(10):2611-27. doi: 10.1113/jphysiol.2012.250431. Epub 2013 Mar 25. J Physiol. 2013. PMID: 23529129 Free PMC article.
-
The entorhinal grid map is discretized.Nature. 2012 Dec 6;492(7427):72-8. doi: 10.1038/nature11649. Nature. 2012. PMID: 23222610
-
Heterosynaptic modulation of evoked synaptic potentials in layer II of the entorhinal cortex by activation of the parasubiculum.J Neurophysiol. 2016 Aug 1;116(2):658-70. doi: 10.1152/jn.00095.2016. Epub 2016 May 4. J Neurophysiol. 2016. PMID: 27146979 Free PMC article.
-
The abrupt development of adult-like grid cell firing in the medial entorhinal cortex.Front Neural Circuits. 2012 Apr 27;6:21. doi: 10.3389/fncir.2012.00021. eCollection 2012. Front Neural Circuits. 2012. PMID: 22557949 Free PMC article.
-
Modeling the Effect of Environmental Geometries on Grid Cell Representations.Front Neural Circuits. 2019 Jan 14;12:120. doi: 10.3389/fncir.2018.00120. eCollection 2018. Front Neural Circuits. 2019. PMID: 30692918 Free PMC article.
References
-
- Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. I. Laminar distribution of theta field potentials. Experimental Brain Research. 1987a;67(3):493–501. - PubMed
-
- Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. II. Phase relations between unit discharges and theta field potentials. Exp Brain Res. 1987b;67(3):502–9. - PubMed
-
- Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol. 1993;70(1):128–43. - PubMed
-
- Alonso A, Llinas RR. Subthreshold Na-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989;342:175–177. - PubMed
-
- Beggs JM, Moyer JR, Jr, McGann JP, Brown TH. Prolonged synaptic integration in perirhinal cortical neurons. J Neurophysiol. 2000;83(6):3294–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

