Amide proton transfer imaging of 9L gliosarcoma and human glioblastoma xenografts
- PMID: 17924591
- PMCID: PMC2943209
- DOI: 10.1002/nbm.1216
Amide proton transfer imaging of 9L gliosarcoma and human glioblastoma xenografts
Abstract
Amide proton transfer (APT) imaging is a variant of magnetization transfer (MT) imaging, in which the contrast is determined by a change in water intensity due to chemical exchange with saturated amide protons of endogenous mobile proteins and peptides. In this study, eight Fisher 344 rats implanted with 9L gliosarcoma cells and six nude rats implanted with human glioblastoma cells were imaged at 4.7 T. There were increased signal intensities in tumors in the APT-weighted images. The contrast of APT imaging between the tumor and contralateral brain tissue was about 3.9% in water intensity (1.49 +/- 0.66% vs -2.36 +/- 0.19%) for the more uniformly hypercellular 9L brain tumors, and it was reduced to 1.6% (-1.18 +/- 0.60% vs -2.77 +/- 0.42%) for the human glioblastoma xenografts that contained hypocellular zones of necrosis. The preliminary results show that the APT technique at the protein level may provide a unique MRI contrast for the characterization of brain tumors.
Copyright (c) 2007 John Wiley & Sons, Ltd.
Figures


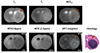
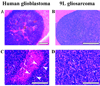
Similar articles
-
Amide proton transfer (APT) contrast for imaging of brain tumors.Magn Reson Med. 2003 Dec;50(6):1120-6. doi: 10.1002/mrm.10651. Magn Reson Med. 2003. PMID: 14648559
-
Amide proton transfer imaging of glioblastoma, neuroblastoma, and breast cancer cells on a 11.7 T magnetic resonance imaging system.Magn Reson Imaging. 2019 Oct;62:181-190. doi: 10.1016/j.mri.2019.07.005. Epub 2019 Jul 11. Magn Reson Imaging. 2019. PMID: 31302222
-
Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semi-solid magnetization transfer reference (EMR) signals: Application to a rat glioma model at 4.7 Tesla.Magn Reson Med. 2016 Jan;75(1):137-49. doi: 10.1002/mrm.25581. Epub 2015 Mar 5. Magn Reson Med. 2016. PMID: 25753614 Free PMC article.
-
Amide Proton Transfer-Chemical Exchange Saturation Transfer Imaging of Intracranial Brain Tumors and Tumor-like Lesions: Our Experience and a Review.Diagnostics (Basel). 2023 Feb 28;13(5):914. doi: 10.3390/diagnostics13050914. Diagnostics (Basel). 2023. PMID: 36900058 Free PMC article. Review.
-
APT-weighted MRI: Techniques, current neuro applications, and challenging issues.J Magn Reson Imaging. 2019 Aug;50(2):347-364. doi: 10.1002/jmri.26645. Epub 2019 Jan 20. J Magn Reson Imaging. 2019. PMID: 30663162 Free PMC article. Review.
Cited by
-
Physics-guided multi-dimensional scan optimization and quasi-steady-state reconstruction to enhance CEST MRI sensitivity efficiency and quantification accuracy.J Magn Reson. 2025 Jan;370:107821. doi: 10.1016/j.jmr.2024.107821. Epub 2024 Dec 12. J Magn Reson. 2025. PMID: 39689390
-
Simultaneous experimental determination of labile proton fraction ratio and exchange rate with irradiation radio frequency power-dependent quantitative CEST MRI analysis.Contrast Media Mol Imaging. 2013 May-Jun;8(3):246-51. doi: 10.1002/cmmi.1524. Contrast Media Mol Imaging. 2013. PMID: 23606428 Free PMC article.
-
Amide chemical exchange saturation transfer at 7 T: a possible biomarker for detecting early response to neoadjuvant chemotherapy in breast cancer patients.Breast Cancer Res. 2018 Jun 14;20(1):51. doi: 10.1186/s13058-018-0982-2. Breast Cancer Res. 2018. PMID: 29898745 Free PMC article. Clinical Trial.
-
Amide proton transfer imaging of the breast at 3 T: establishing reproducibility and possible feasibility assessing chemotherapy response.Magn Reson Med. 2013 Jul;70(1):216-24. doi: 10.1002/mrm.24450. Epub 2012 Aug 20. Magn Reson Med. 2013. PMID: 22907893 Free PMC article.
-
Amide proton transfer imaging to discriminate between low- and high-grade gliomas: added value to apparent diffusion coefficient and relative cerebral blood volume.Eur Radiol. 2017 Aug;27(8):3181-3189. doi: 10.1007/s00330-017-4732-0. Epub 2017 Jan 23. Eur Radiol. 2017. PMID: 28116517 Free PMC article.
References
-
- Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn. Reson. Med. 1989;10:135–144. - PubMed
-
- Balaban RS, Ceckler TL. Magnetization transfer contrast in magnetic resonance imaging. Magn. Reson. Q. 1992;8:116–137. - PubMed
-
- Bryant RG. The dynamics of water-protein interactions. Annu. Rev. Biophys. Biomol. Struct. 1996;25:29–53. - PubMed
-
- Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR Biomed. 2001;14:57–64. - PubMed
-
- Yousem DM, Montone KT, Sheppard LM, Rao VM, Weinstein GS, Hayden RE. Head and neck neoplasms - magnetization-transfer analysis. Radiology. 1994;192:703–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

