Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis
- PMID: 17924976
- PMCID: PMC11159678
- DOI: 10.1111/j.1349-7006.2007.00633.x
Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis
Abstract
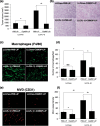
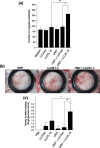
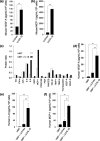
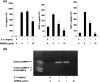
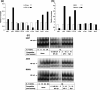
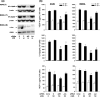
The focus of the present study was whether and how infiltrating macrophages play a role in angiogenesis and the growth of cancer cells in response to the inflammatory cytokine interleukin (IL)-1beta. Lewis lung carcinoma cells overexpressing IL-1beta grew faster and induced greater neovascularization than a low IL-1beta-expressing counterpart in vivo. When macrophages were depleted using clodronate liposomes, both neovascularization and tumor growth were reduced in the IL-1beta-expressing tumors. Co-cultivation of IL-1beta-expressing cancer cells with macrophages synergistically augmented neovascularization and the migration of vascular endothelial cells. In these co-cultures, production of the angiogenic factors vascular endothelial growth factor-A and IL-8, monocyte chemoattractant protein-1, and matrix metalloproteinase-9 were increased markedly. The production of these factors, induced by IL-1beta-stimulated lung cancer cells, was blocked by a nuclear factor (NF)-kappaB inhibitor, and also by the knockdown of p65 (NF-kappaB) and c-Jun using small interference RNA, suggesting involvement of the transcription factors NF-kappaB and AP-1. These results demonstrated that macrophages recruited into tumors by monocyte chemoattractant protein-1 and other chemokines could play a critical role in promoting tumor growth and angiogenesis, through interactions with cancer cells mediated by inflammatory stimuli.
Figures
Similar articles
-
Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth.J Clin Invest. 2005 Nov;115(11):2979-91. doi: 10.1172/JCI23298. Epub 2005 Oct 20. J Clin Invest. 2005. PMID: 16239969 Free PMC article.
-
Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy.Cancer Sci. 2008 Aug;99(8):1501-6. doi: 10.1111/j.1349-7006.2008.00853.x. Cancer Sci. 2008. PMID: 18754859 Free PMC article. Review.
-
N-myc downstream-regulated gene 1 promotes tumor inflammatory angiogenesis through JNK activation and autocrine loop of interleukin-1α by human gastric cancer cells.J Biol Chem. 2013 Aug 30;288(35):25025-25037. doi: 10.1074/jbc.M113.472068. Epub 2013 Jul 11. J Biol Chem. 2013. PMID: 23846687 Free PMC article.
-
Multifunctional interleukin-1beta promotes metastasis of human lung cancer cells in SCID mice via enhanced expression of adhesion-, invasion- and angiogenesis-related molecules.Cancer Sci. 2003 Mar;94(3):244-52. doi: 10.1111/j.1349-7006.2003.tb01428.x. Cancer Sci. 2003. PMID: 12824917 Free PMC article.
-
Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer.Clin Cancer Res. 2003 Feb;9(2):729-37. Clin Cancer Res. 2003. PMID: 12576442
Cited by
-
Nuclear expression of N-myc downstream regulated gene 1/Ca(2+)-associated protein 43 is closely correlated with tumor angiogenesis and poor survival in patients with gastric cancer.Exp Ther Med. 2011 May;2(3):471-479. doi: 10.3892/etm.2011.222. Epub 2011 Mar 2. Exp Ther Med. 2011. PMID: 22977527 Free PMC article.
-
Pivotal Role of Cranial Irradiation-Induced Peripheral, Intrinsic, and Brain-Engrafting Macrophages in Malignant Glioma.Clin Med Insights Oncol. 2024 Oct 12;18:11795549241282098. doi: 10.1177/11795549241282098. eCollection 2024. Clin Med Insights Oncol. 2024. PMID: 39421649 Free PMC article. Review.
-
Tumour hypoxia promotes melanoma growth and metastasis via High Mobility Group Box-1 and M2-like macrophages.Sci Rep. 2016 Jul 18;6:29914. doi: 10.1038/srep29914. Sci Rep. 2016. PMID: 27426915 Free PMC article.
-
Nitric Oxide Generated by Tumor-Associated Macrophages Is Responsible for Cancer Resistance to Cisplatin and Correlated With Syntaxin 4 and Acid Sphingomyelinase Inhibition.Front Immunol. 2018 May 29;9:1186. doi: 10.3389/fimmu.2018.01186. eCollection 2018. Front Immunol. 2018. PMID: 29896202 Free PMC article.
-
Liposomal clodronate treatment for tumour macrophage depletion in dogs with soft-tissue sarcoma.Vet Comp Oncol. 2013 Dec;11(4):296-305. doi: 10.1111/j.1476-5829.2012.00319.x. Epub 2012 Apr 27. Vet Comp Oncol. 2013. PMID: 22540967 Free PMC article.
References
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357: 539–45. - PubMed
-
- Pollard JW. Tumour‐educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4: 71–8. - PubMed
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med 2003; 9: 653–60. - PubMed
-
- Kuwano M, Basaki Y, Kuwano T et al . The critical role of inflammatory cell infiltration in tumor angiogenesis – a target for antitumor drug development? In: New Angiogenesis Research. New York: Nova Science Publishers, 2005: 157–70.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

