Inhibition of neutrophil activity improves cardiac function after cardiopulmonary bypass
- PMID: 17925040
- PMCID: PMC2100046
- DOI: 10.1186/1476-9255-4-21
Inhibition of neutrophil activity improves cardiac function after cardiopulmonary bypass
Abstract
Background: The arterial in line application of the leukocyte inhibition module (LIM) in the cardiopulmonary bypass (CPB) limits overshooting leukocyte activity during cardiac surgery. We studied in a porcine model whether LIM may have beneficial effects on cardiac function after CPB.
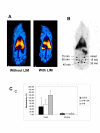
Methods: German landrace pigs underwent CPB (60 min myocardial ischemia; 30 min reperfusion) without (group I; n = 6) or with LIM (group II; n = 6). The cardiac indices (CI) and cardiac function were analyzed pre and post CPB with a Swan-Ganz catheter and the cardiac function analyzer. Neutrophil labeling with technetium, scintigraphy, and histological analyses were done to track activated neutrophils within the organs.
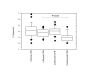
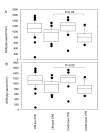
Results: LIM prevented CPB-associated increase of neutrophil counts in peripheral blood. In group I, the CI significantly declined post CPB (post: 3.26 +/- 0.31; pre: 4.05 +/- 0.45 l/min/m2; p < 0.01). In group II, the CI was only slightly reduced (post: 3.86 +/- 0.49; pre 4.21 +/- 1.32 l/min/m2; p = 0.23). Post CPB, the intergroup difference showed significantly higher CI values in the LIM group (p < 0.05) which was in conjunction with higher pre-load independent endsystolic pressure volume relationship (ESPVR) values (group I: 1.57 +/- 0.18; group II: 1.93 +/- 0.16; p < 0.001). Moreover, the systemic vascular resistance and pulmonary vascular resistance were lower in the LIM group. LIM appeared to accelerate the sequestration of hyperactivated neutrophils in the spleen and to reduce neutrophil infiltration of heart and lung.
Conclusion: Our data provides strong evidence that LIM improves perioperative hemodynamics and cardiac function after CPB by limiting neutrophil activity and inducing accelerated sequestration of neutrophils in the spleen.
Figures



Similar articles
-
[The influence of cardiopulmonary bypass operation on the biodistribution of 99mTc-HMPAO-labelled granulocytes - Evaluation in pigs by planar scintigraphy and section-analyses].Nuklearmedizin. 2012;51(5):205-11. doi: 10.3413/Nukmed-0434-11-10. Epub 2012 May 29. Nuklearmedizin. 2012. PMID: 22641340 German.
-
In vivo inhibition of neutrophil activity by a FAS (CD95) stimulating module: arterial in-line application in a porcine cardiac surgery model.J Thorac Cardiovasc Surg. 2004 Jun;127(6):1735-42. doi: 10.1016/j.jtcvs.2003.09.027. J Thorac Cardiovasc Surg. 2004. PMID: 15173731
-
Does a hyperoncotic cardiopulmonary bypass prime affect extravascular lung water and cardiopulmonary function in patients undergoing coronary artery bypass surgery?Eur J Cardiothorac Surg. 2001 Aug;20(2):282-9. doi: 10.1016/s1010-7940(01)00804-1. Eur J Cardiothorac Surg. 2001. PMID: 11463545 Clinical Trial.
-
Inhibition of neutrophil activity in cardiac surgery with cardiopulmonary bypass: a novel strategy with the leukocyte inhibition module.Perfusion. 2004 Jan;19(1):11-6. doi: 10.1191/0267659104pf709oa. Perfusion. 2004. PMID: 15072250 Review.
-
Cardiopulmonary bypass-induced inflammation: is it important?J Cardiothorac Vasc Anesth. 1998 Apr;12(2 Suppl 1):21-5. J Cardiothorac Vasc Anesth. 1998. PMID: 9583572 Review.
Cited by
-
Current Understanding of Leukocyte Phenotypic and Functional Modulation During Extracorporeal Membrane Oxygenation: A Narrative Review.Front Immunol. 2021 Jan 8;11:600684. doi: 10.3389/fimmu.2020.600684. eCollection 2020. Front Immunol. 2021. PMID: 33488595 Free PMC article. Review.
-
Extracorporeal immune therapy with immobilized agonistic anti-Fas antibodies leads to transient reduction of circulating neutrophil numbers and limits tissue damage after hemorrhagic shock/resuscitation in a porcine model.J Inflamm (Lond). 2010 Apr 20;7:18. doi: 10.1186/1476-9255-7-18. J Inflamm (Lond). 2010. PMID: 20406470 Free PMC article.
-
Anethole and eugenol reduce in vitro and in vivo leukocyte migration induced by fMLP, LTB4, and carrageenan.J Nat Med. 2014 Jul;68(3):567-75. doi: 10.1007/s11418-014-0839-7. Epub 2014 May 1. J Nat Med. 2014. PMID: 24789168
-
Optimal intervention time of vagal stimulation attenuating myocardial ischemia/reperfusion injury in rats.Inflamm Res. 2014 Dec;63(12):987-99. doi: 10.1007/s00011-014-0775-8. Epub 2014 Oct 8. Inflamm Res. 2014. PMID: 25292223
-
Increased expression of cardiac IL-17 after burn.J Inflamm (Lond). 2010 Jul 27;7:38. doi: 10.1186/1476-9255-7-38. J Inflamm (Lond). 2010. PMID: 20663214 Free PMC article.
References
-
- Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983;86:845–857. - PubMed
-
- Butler J, Rocker GM, Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993;55:552–559. - PubMed
-
- Carden D, Xiao F, Moak C, Willis BH, Robinson-Jackson S, Alexander S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am J Physiol. 1998;275:H385–H392. - PubMed
-
- Welbourn CR, Goldman G, Paterson IS, Valeri CR, Shepro D, Hechtman HB. Neutrophil elastase and oxygen radicals: synergism in lung injury after hindlimb ischemia. Am J Physiol. 1991;260:H1852–H1856. - PubMed
LinkOut - more resources
Full Text Sources

