Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation
- PMID: 17925248
- PMCID: PMC2408750
- DOI: 10.1016/S0079-6123(06)65013-9
Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation
Abstract
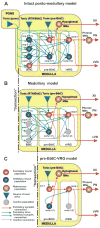
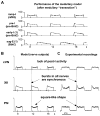
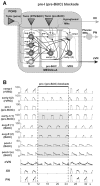
The brainstem respiratory network can operate in multiple functional states engaging different state-dependent neural mechanisms. These mechanisms were studied in the in situ perfused rat brainstem-spinal cord preparation using sequential brainstem transections and administration of riluzole, a pharmacological blocker of persistent sodium current (INaP). Dramatic transformations in the rhythmogenic mechanisms and respiratory motor pattern were observed after removal of the pons and subsequent medullary transactions down to the rostral end of pre-Bötzinger complex (pre-BötC). A computational model of the brainstem respiratory network was developed to reproduce and explain these experimental findings. The model incorporates several interacting neuronal compartments, including the ventral respiratory group (VRG), pre-BötC, Bötzinger complex (BötC), and pons. Simulations mimicking the removal of circuit components following transections closely reproduce the respiratory motor output patterns recorded from the intact and sequentially reduced brainstem preparations. The model suggests that both the operating rhythmogenic mechanism (i.e., network-based or pacemaker-driven) and the respiratory pattern generated (e.g., three-phase, two-phase, or one-phase) depend on the state of the pre-BötC (expression of INaP-dependent intrinsic rhythmogenic mechanisms) and the BötC (providing expiratory inhibition in the network). At the same time, tonic drives from pons and multiple medullary chemoreceptive sites appear to control the state of these compartments and hence the operating rhythmogenic mechanism and motor pattern. Our results suggest that the brainstem respiratory network has a spatial (rostral-to-caudal) organization extending from the rostral pons to the VRG, in which each functional compartment is controlled by more rostral compartments. The model predicts a continuum of respiratory network states relying on different contributions of intrinsic cellular properties versus synaptic interactions for the generation and control of the respiratory rhythm and pattern.
Figures




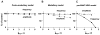
References
-
- Abdala APL, Koizumi H, Rybak IA, Smith JC, Paton JFR. The 3-2-1 state respiratory rhythm generator hypothesis revealed by microsectioning, reduced extracellular chloride and alterations in arterial gas tensions in the in situ rat. Exp Biology Abstr. 2007:610.4.
-
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol. 2004;143:105–114. - PubMed
-
- Butera RJ, Rinzel JR, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex: I. Bursting pacemaker neurons. J Neurophysiol. 1999a;82:382–397. - PubMed
-
- Butera RJ, Rinzel JR, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex: II. Populations of coupled pacemaker neurons. J Neurophysiol. 1999b;82:398–415. - PubMed
-
- Cohen MI. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979;59:1105–1173. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

