In vitro dimerization of human immunodeficiency virus type 1 (HIV-1) spliced RNAs
- PMID: 17925344
- PMCID: PMC2080610
- DOI: 10.1261/rna.678307
In vitro dimerization of human immunodeficiency virus type 1 (HIV-1) spliced RNAs
Abstract
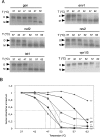
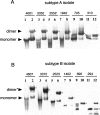
The human immunodeficiency virus type 1 (HIV-1) packages its genomic RNA as a dimer of homologous RNA molecules that has to be selected among a multitude of cellular and viral RNAs. Interestingly, spliced viral mRNAs are packaged into viral particles with a relatively low efficiency despite the fact that they contain most of the extended packaging signal found in the 5' untranslated region of the genomic RNA, including the dimerization initiation site (DIS). As a consequence, HIV-1 spliced viral RNAs can theoretically homodimerize and heterodimerize with the genomic RNA, and thus they should directly compete with genomic RNA for packaging. To shed light on this issue, we investigated for the first time the in vitro dimerization properties of spliced HIV-1 RNAs. We found that singly spliced (env, vpr) and multispliced (tat, rev, and nef) RNA fragments are able to dimerize in vitro, and to efficiently form heterodimers with genomic RNA. Chemical probing experiments and inhibition of RNA dimerization by an antisense oligonucleotide directed against the DIS indicated that the DIS is structurally functional in spliced HIV-1 RNA, and that RNA dimerization occurs through a loop-loop interaction. In addition, by combining in vitro transcription and dimerization assays, we show that heterodimers can be efficiently formed only when the two RNA fragments are synthesized simultaneously, in the same environment. Together, our results support a model in which RNA dimerization would occur during transcription in the nucleus and could thus play a major role in splicing, transport, and localization of HIV-1 RNA.
Figures


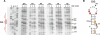

References
-
- Balakrishnan, M., Fay, P.J., Bambara, R.A. The kissing hairpin sequence promotes recombination within the HIV-I 5′ leader region. J. Biol. Chem. 2001;276:36482–36492. - PubMed
-
- Basyuk, E., Boulon, S., Skou Pedersen, F., Bertrand, E., Vestergaard Rasmussen, S. The packaging signal of MLV is an integrated module that mediates intracellular transport of genomic RNAs. J. Mol. Biol. 2005;354:330–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
