A small-molecule therapeutic lead for Huntington's disease: preclinical pharmacology and efficacy of C2-8 in the R6/2 transgenic mouse
- PMID: 17925440
- PMCID: PMC2034257
- DOI: 10.1073/pnas.0707842104
A small-molecule therapeutic lead for Huntington's disease: preclinical pharmacology and efficacy of C2-8 in the R6/2 transgenic mouse
Abstract
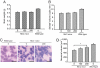
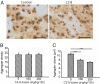
Huntington's disease (HD) is a progressive neurodegenerative disease caused by a glutamine expansion within huntingtin protein. The exact pathological mechanisms determining disease onset and progression remain unclear. However, aggregates of insoluble mutant huntingtin (mhtt), a hallmark of HD, are readily detected within neurons in HD brain. Although aggregated polyglutamines may not be inherently toxic, they constitute a biomarker for mutant huntingtin useful for developing therapeutics. We previously reported that the small molecule, C2-8, inhibits polyglutamine aggregation in cell culture and brain slices and rescues degeneration of photoreceptors in a Drosophila model of HD. In this study, we assessed the therapeutic potential of C2-8 in the R6/2 mouse model of HD, which has been used to provide proof-of-concept data in considering whether to advance therapies to human HD. We show that, at nontoxic doses, C2-8 penetrates the blood-brain barrier and is present in brain at a high concentration. C2-8-treated mice showed improved motor performance and reduced neuronal atrophy and had smaller huntingtin aggregates. There have been no prior drug-like, non-toxic, brain-penetrable aggregation inhibitors to arise from cell-based high-throughput screens for reducing huntingtin aggregation that is efficacious in preclinical in vivo models. C2-8 provides an essential tool to help elucidate mechanisms of neurodegeneration in HD and a therapeutic lead for further optimization and development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

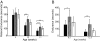
References
-
- The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–83. - PubMed
-
- Gusella JF, MacDonald ME. Nat Rev Neurosci. 2000;1:109–15. - PubMed
-
- Bates G, Harper P, Jones L. Huntington's Disease. New York: Oxford Univ Press; 2002.
-
- Hersch SM, Rosas HR, Ferrante RJ. In: Movement Disorders: Neuropathologic Principles and Practice. Watta RL, Koller WC, editors. New York: McGraw–Hill; 2004. pp. 503–523.
-
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Cell. 1996;87:493–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Medical
Miscellaneous

