Longitudinally propagating traveling waves of the mammalian tectorial membrane
- PMID: 17925447
- PMCID: PMC2034249
- DOI: 10.1073/pnas.0703665104
Longitudinally propagating traveling waves of the mammalian tectorial membrane
Abstract
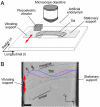
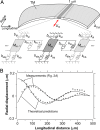
Sound-evoked vibrations transmitted into the mammalian cochlea produce traveling waves that provide the mechanical tuning necessary for spectral decomposition of sound. These traveling waves of motion that have been observed to propagate longitudinally along the basilar membrane (BM) ultimately stimulate the mechano-sensory receptors. The tectorial membrane (TM) plays a key role in this process, but its mechanical function remains unclear. Here we show that the TM supports traveling waves that are an intrinsic feature of its visco-elastic structure. Radial forces applied at audio frequencies (2-20 kHz) to isolated TM segments generate longitudinally propagating waves on the TM with velocities similar to those of the BM traveling wave near its best frequency place. We compute the dynamic shear storage modulus and shear viscosity of the TM from the propagation velocity of the waves and show that segments of the TM from the basal turn are stiffer than apical segments are. Analysis of loading effects of hair bundle stiffness, the limbal attachment of the TM, and viscous damping in the subtectorial space suggests that TM traveling waves can occur in vivo. Our results show the presence of a traveling wave mechanism through the TM that can functionally couple a significant longitudinal extent of the cochlea and may interact with the BM wave to greatly enhance cochlear sensitivity and tuning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dallos P. In: The Cochlea. Dallos P, Popper AN, Fay RR, editors. New York: Springer; 1996. pp. 1–43.
-
- von Békésy G. Experiments in Hearing. New York: McGraw–Hill; 1960.
-
- Hudspeth AJ. Science. 1985;230:745–752. - PubMed
-
- McGuirt WT, Prasad SD, Griffith AJ, Kunst H, Green GE, Shpargel KB, Runge C, Huybrechts C, Mueller RF, et al. Nat Genet. 1999;23:413–419. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

