Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children's Oncology Group
- PMID: 17925486
- PMCID: PMC2265463
- DOI: 10.1182/blood-2007-08-106286
Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children's Oncology Group
Abstract
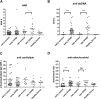
Numerous chronic graft-versus-host disease (cGVHD) biomarkers have been identified in limited, single-institution studies without validation. We hypothesized that plasma-derived biomarkers could diagnose, classify, and evaluate response in children with cGVHD. We performed a concomitant analysis of a number of known and predicted peripheral blood cGVHD biomarkers from a Children's Oncology Group (COG) phase 3 cGVHD therapeutic trial. A total of 52 newly diagnosed patients with extensive cGVHD were compared for time of onset after blood and marrow transplantation (BMT) (early, 3-8 months; late, > or = 9 months) with 28 time-matched controls with no cGVHD (early, 6 months after BMT; late, 12 months after BMT). Soluble B-cell activation factor (sBAFF), anti-dsDNA antibody, soluble IL-2 receptor alpha (sIL-2Ralpha), and soluble CD13 (sCD13) were elevated in patients with early-onset cGVHD compared with controls. sBAFF and anti-dsDNA were elevated in patients with late-onset cGVHD. Some of the biomarkers correlated with specific organ involvement and with therapeutic response. These 4 biomarkers had high specificity with higher sensitivity in combination. Changes in biomarker concentrations with immune reconstitution after transplantation significantly affected interpretation of results. The identified biomarkers have the potential for improved classification, early response evaluation, and direction of cGVHD treatment, but require validation in larger studies. This study is registered at www.cancer.gov/clinicaltrials as no. COG-ASCT0031.
Figures




References
-
- Remberger M, Beelen DW, Fauser A, et al. Increased risk of extensive chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation using unrelated donors. Blood. 2005;105:548–551. - PubMed
-
- Kollman C, Howe CWS, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. - PubMed
-
- Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versushost disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. - PubMed
-
- Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

