Tsukushi modulates Xnr2, FGF and BMP signaling: regulation of Xenopus germ layer formation
- PMID: 17925852
- PMCID: PMC1994590
- DOI: 10.1371/journal.pone.0001004
Tsukushi modulates Xnr2, FGF and BMP signaling: regulation of Xenopus germ layer formation
Abstract
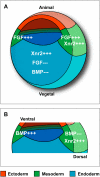
Background: Cell-cell communication is essential in tissue patterning. In early amphibian development, mesoderm is formed in the blastula-stage embryo through inductive interactions in which vegetal cells act on overlying equatorial cells. Members of the TGF-beta family such as activin B, Vg1, derrière and Xenopus nodal-related proteins (Xnrs) are candidate mesoderm inducing factors, with further activity to induce endoderm of the vegetal region. TGF-beta-like ligands, including BMP, are also responsible for patterning of germ layers. In addition, FGF signaling is essential for mesoderm formation whereas FGF signal inhibition has been implicated in endoderm induction. Clearly, several signaling pathways are coordinated to produce an appropriate developmental output; although intracellular crosstalk is known to integrate multiple pathways, relatively little is known about extracellular coordination.
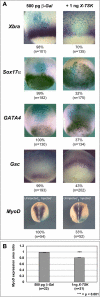
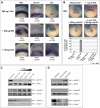
Methodology/principal findings: Here, we show that Xenopus Tsukushi (X-TSK), a member of the secreted small leucine rich repeat proteoglycan (SLRP) family, is expressed in ectoderm, endoderm, and the organizer during early development. We have previously reported that X-TSK binds to and inhibits BMP signaling in cooperation with chordin. We now demonstrate two novel interactions: X-TSK binds to and inhibits signaling by FGF8b, in addition to binding to and enhancement of Xnr2 signaling. This signal integration by X-TSK at the extracellular level has an important role in germ layer formation and patterning. Vegetally localized X-TSK potentiates endoderm formation through coordination of BMP, FGF and Xnr2 signaling. In contrast, X-TSK inhibition of FGF-MAPK signaling blocks ventrolateral mesoderm formation, while BMP inhibition enhances organizer formation. These actions of X-TSK are reliant upon its expression in endoderm and dorsal mesoderm, with relative exclusion from ventrolateral mesoderm, in a pattern shaped by FGF signals.
Conclusions/significance: Based on our observations, we propose a novel mechanism by which X-TSK refines the field of positional information by integration of multiple pathways in the extracellular space.
Conflict of interest statement
Figures

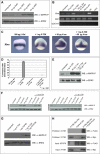
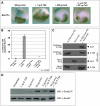
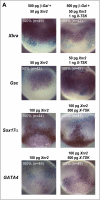
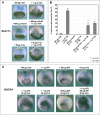
Similar articles
-
Two-step induction of primitive erythrocytes in Xenopus laevis embryos: signals from the vegetal endoderm and the overlying ectoderm.Int J Dev Biol. 2001 Apr;45(2):387-96. Int J Dev Biol. 2001. PMID: 11330858
-
Tbx2 mediates dorsal patterning and germ layer suppression through inhibition of BMP/GDF and Activin/Nodal signaling.BMC Mol Cell Biol. 2020 May 28;21(1):39. doi: 10.1186/s12860-020-00282-1. BMC Mol Cell Biol. 2020. PMID: 32466750 Free PMC article.
-
Zebrafish endoderm formation is regulated by combinatorial Nodal, FGF and BMP signalling.Development. 2006 Jun;133(11):2189-200. doi: 10.1242/dev.02387. Epub 2006 May 3. Development. 2006. PMID: 16672336
-
Dorsal-ventral patterning and neural induction in Xenopus embryos.Annu Rev Cell Dev Biol. 2004;20:285-308. doi: 10.1146/annurev.cellbio.20.011403.154124. Annu Rev Cell Dev Biol. 2004. PMID: 15473842 Free PMC article. Review.
-
Pluripotent cells (stem cells) and their determination and differentiation in early vertebrate embryogenesis.Dev Growth Differ. 2001 Oct;43(5):469-502. doi: 10.1046/j.1440-169x.2001.00599.x. Dev Growth Differ. 2001. PMID: 11576166 Review.
Cited by
-
The matricellular functions of small leucine-rich proteoglycans (SLRPs).J Cell Commun Signal. 2009 Dec;3(3-4):323-35. doi: 10.1007/s12079-009-0066-2. Epub 2009 Oct 2. J Cell Commun Signal. 2009. PMID: 19809894 Free PMC article.
-
A novel fibroblast growth factor receptor family member promotes neuronal outgrowth and synaptic plasticity in aplysia.Amino Acids. 2014 Nov;46(11):2477-88. doi: 10.1007/s00726-014-1803-2. Epub 2014 Jul 25. Amino Acids. 2014. PMID: 25059541 Free PMC article.
-
Tsukushi is essential for the formation of the posterior semicircular canal that detects gait performance.J Cell Commun Signal. 2021 Dec;15(4):581-594. doi: 10.1007/s12079-021-00627-1. Epub 2021 Jun 1. J Cell Commun Signal. 2021. PMID: 34061311 Free PMC article.
-
Involvement of Tsukushi in diverse developmental processes.J Cell Commun Signal. 2018 Mar;12(1):205-210. doi: 10.1007/s12079-018-0452-8. Epub 2018 Jan 19. J Cell Commun Signal. 2018. PMID: 29352451 Free PMC article.
-
Two Secreted Proteoglycans, Activators of Urothelial Cell-Cell Adhesion, Negatively Contribute to Bladder Cancer Initiation and Progression.Cancers (Basel). 2020 Nov 13;12(11):3362. doi: 10.3390/cancers12113362. Cancers (Basel). 2020. PMID: 33202923 Free PMC article.
References
-
- Heasman J. Patterning the Xenopus blastula. Development. 1997;124:4179–4191. - PubMed
-
- Darras S, Marikawa Y, Elinson RP, Lemaire P. Animal and vegetal pole cells of early Xenopus embryos respond differently to maternal dorsal determinants: implications for the patterning of the organiser. Development. 1997;124:4275–4286. - PubMed
-
- Loose M, Patient R. A genetic regulatory network for Xenopus mesendoderm formation. Dev Biol. 2004;271:467–478. - PubMed
-
- Smith JC, Price BM, Nimmen KV, Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990;345:729–731. - PubMed
-
- Weeks DL, Melton DA. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987;51:861–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

