SIRNA-directed in vivo silencing of androgen receptor inhibits the growth of castration-resistant prostate carcinomas
- PMID: 17925854
- PMCID: PMC1994591
- DOI: 10.1371/journal.pone.0001006
SIRNA-directed in vivo silencing of androgen receptor inhibits the growth of castration-resistant prostate carcinomas
Abstract
Background: Prostate carcinomas are initially dependent on androgens, and castration or androgen antagonists inhibit their growth. After some time though, tumors become resistant and recur with a poor prognosis. The majority of resistant tumors still expresses a functional androgen receptor (AR), frequently amplified or mutated.
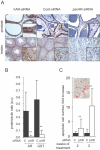
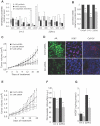
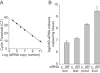
Methodology/principal findings: To test the hypothesis that AR is not only expressed, but is still a key therapeutic target in advanced carcinomas, we injected siRNA targeting AR into mice bearing exponentially growing castration-resistant tumors. Quantification of siRNA into tumors and mouse tissues demonstrated their efficient uptake. This uptake silenced AR in the prostate, testes and tumors. AR silencing in tumors strongly inhibited their growth, and importantly, also markedly repressed the VEGF production and angiogenesis.
Conclusions/significance: Our results demonstrate that carcinomas resistant to hormonal manipulations still depend on the expression of the androgen receptor for their development in vivo. The siRNA-directed silencing of AR, which allows targeting overexpressed as well as mutated isoforms, triggers a strong antitumoral and antiangiogenic effect. siRNA-directed silencing of this key gene in advanced and resistant prostate tumors opens promising new therapeutic perspectives and tools.
Conflict of interest statement
Figures

Similar articles
-
siRNA Lipid Nanoparticle Potently Silences Clusterin and Delays Progression When Combined with Androgen Receptor Cotargeting in Enzalutamide-Resistant Prostate Cancer.Clin Cancer Res. 2015 Nov 1;21(21):4845-55. doi: 10.1158/1078-0432.CCR-15-0866. Epub 2015 Jun 23. Clin Cancer Res. 2015. PMID: 26106075
-
In vivo knockdown of the androgen receptor results in growth inhibition and regression of well-established, castration-resistant prostate tumors.Clin Cancer Res. 2009 Jan 1;15(1):39-47. doi: 10.1158/1078-0432.CCR-08-1726. Clin Cancer Res. 2009. PMID: 19118031
-
Anti-tumor effect of small interfering RNA targeting the androgen receptor in human androgen-independent prostate cancer cells.Biochem Biophys Res Commun. 2010 Jan 1;391(1):1075-9. doi: 10.1016/j.bbrc.2009.12.024. Epub 2009 Dec 14. Biochem Biophys Res Commun. 2010. PMID: 20004643
-
Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer.Free Radic Biol Med. 2011 Oct 1;51(7):1320-8. doi: 10.1016/j.freeradbiomed.2011.07.011. Epub 2011 Jul 23. Free Radic Biol Med. 2011. PMID: 21820046 Review.
-
Androgen action in the prostate gland.Minerva Urol Nefrol. 2012 Mar;64(1):35-49. Minerva Urol Nefrol. 2012. PMID: 22402316 Review.
Cited by
-
Modulation of androgen receptor signaling in hormonal therapy-resistant prostate cancer cell lines.PLoS One. 2011;6(8):e23144. doi: 10.1371/journal.pone.0023144. Epub 2011 Aug 4. PLoS One. 2011. PMID: 21829708 Free PMC article.
-
Prostate-targeted biodegradable nanoparticles loaded with androgen receptor silencing constructs eradicate xenograft tumors in mice.Nanomedicine (Lond). 2012 Sep;7(9):1297-309. doi: 10.2217/nnm.12.14. Epub 2012 May 14. Nanomedicine (Lond). 2012. PMID: 22583574 Free PMC article.
-
CEACAM6 attenuates adenovirus infection by antagonizing viral trafficking in cancer cells.J Clin Invest. 2009 Jun;119(6):1604-15. doi: 10.1172/JCI37905. J Clin Invest. 2009. PMID: 19411761 Free PMC article.
-
Androgen receptor activation in castration-recurrent prostate cancer: the role of Src-family and Ack1 tyrosine kinases.Int J Biol Sci. 2014 Jun 5;10(6):620-6. doi: 10.7150/ijbs.8264. eCollection 2014. Int J Biol Sci. 2014. PMID: 24948875 Free PMC article. Review.
-
Anti-Androgen Receptor Therapies in Prostate Cancer: A Brief Update and Perspective.Front Oncol. 2022 Mar 10;12:865350. doi: 10.3389/fonc.2022.865350. eCollection 2022. Front Oncol. 2022. PMID: 35372068 Free PMC article. Review.
References
-
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. - PubMed
-
- Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. - PubMed
-
- Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, et al. Intraprostatic Androgens and Androgen-Regulated Gene Expression Persist after Testosterone Suppression: Therapeutic Implications for Castration-Resistant Prostate Cancer. Cancer Res. 2007;67:5033–5041. - PubMed
-
- Culig Z, Steiner H, Bartsch G, Hobisch A. Mechanisms of endocrine therapy-responsive and -unresponsive prostate tumours. Endocr Relat Cancer. 2005;12:229–244. - PubMed
-
- Scher HI, Sawyers CL. Biology of Progressive, Castration-Resistant Prostate Cancer: Directed Therapies Targeting the Androgen-Receptor Signaling Axis. J Clin Oncol. 2005;23:8253–8261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

