Sensitive detection of p65 homodimers using red-shifted and fluorescent protein-based FRET couples
- PMID: 17925859
- PMCID: PMC1995760
- DOI: 10.1371/journal.pone.0001011
Sensitive detection of p65 homodimers using red-shifted and fluorescent protein-based FRET couples
Abstract
Background: Fluorescence Resonance Energy Transfer (FRET) between the green fluorescent protein (GFP) variants CFP and YFP is widely used for the detection of protein-protein interactions. Nowadays, several monomeric red-shifted fluorescent proteins are available that potentially improve the efficiency of FRET.
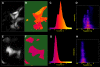
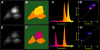
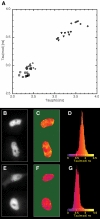
Methodology/principal findings: To allow side-by-side comparison of several fluorescent protein combinations for detection of FRET, yellow or orange fluorescent proteins were directly fused to red fluorescent proteins. FRET from yellow fluorescent proteins to red fluorescent proteins was detected by both FLIM and donor dequenching upon acceptor photobleaching, showing that mCherry and mStrawberry were more efficient acceptors than mRFP1. Circular permutated yellow fluorescent protein variants revealed that in the tandem constructs the orientation of the transition dipole moment influences the FRET efficiency. In addition, it was demonstrated that the orange fluorescent proteins mKO and mOrange are both suitable as donor for FRET studies. The most favorable orange-red FRET pair was mKO-mCherry, which was used to detect homodimerization of the NF-kappaB subunit p65 in single living cells, with a threefold higher lifetime contrast and a twofold higher FRET efficiency than for CFP-YFP.
Conclusions/significance: The observed high FRET efficiency of red-shifted couples is in accordance with increased Förster radii of up to 64 A, being significantly higher than the Förster radius of the commonly used CFP-YFP pair. Thus, red-shifted FRET pairs are preferable for detecting protein-protein interactions by donor-based FRET methods in single living cells.
Conflict of interest statement
Figures

Similar articles
-
Flow cytometric measurement of fluorescence (Förster) resonance energy transfer from cyan fluorescent protein to yellow fluorescent protein using single-laser excitation at 458 nm.Cytometry A. 2003 May;53(1):39-54. doi: 10.1002/cyto.a.10037. Cytometry A. 2003. PMID: 12701131
-
Quantitative comparison of different fluorescent protein couples for fast FRET-FLIM acquisition.Biophys J. 2009 Oct 21;97(8):2368-76. doi: 10.1016/j.bpj.2009.07.044. Biophys J. 2009. PMID: 19843469 Free PMC article.
-
Sensitivity of CFP/YFP and GFP/mCherry pairs to donor photobleaching on FRET determination by fluorescence lifetime imaging microscopy in living cells.Microsc Res Tech. 2006 Nov;69(11):933-9. doi: 10.1002/jemt.20370. Microsc Res Tech. 2006. PMID: 16941642
-
Fanciful FRET.Sci STKE. 2006 Apr 18;2006(331):re2. doi: 10.1126/stke.3312006re2. Sci STKE. 2006. PMID: 16622184 Review.
-
Advances in fluorescent protein technology.J Cell Sci. 2007 Dec 15;120(Pt 24):4247-60. doi: 10.1242/jcs.005801. J Cell Sci. 2007. PMID: 18057027 Review.
Cited by
-
Chromophore transformations in red fluorescent proteins.Chem Rev. 2012 Jul 11;112(7):4308-27. doi: 10.1021/cr2001965. Epub 2012 May 4. Chem Rev. 2012. PMID: 22559232 Free PMC article. Review. No abstract available.
-
Positive control of cell division: FtsZ is recruited by SsgB during sporulation of Streptomyces.Genes Dev. 2011 Jan 1;25(1):89-99. doi: 10.1101/gad.600211. Genes Dev. 2011. PMID: 21205868 Free PMC article.
-
Single-cell imaging of mechanotransduction in endothelial cells.Prog Mol Biol Transl Sci. 2014;126:25-51. doi: 10.1016/B978-0-12-394624-9.00002-6. Prog Mol Biol Transl Sci. 2014. PMID: 25081613 Free PMC article. Review.
-
Characterization of a spectrally diverse set of fluorescent proteins as FRET acceptors for mTurquoise2.Sci Rep. 2017 Sep 20;7(1):11999. doi: 10.1038/s41598-017-12212-x. Sci Rep. 2017. PMID: 28931898 Free PMC article.
-
Multiplexed FRET to image multiple signaling events in live cells.Biophys J. 2008 Nov 15;95(10):L69-71. doi: 10.1529/biophysj.108.139204. Epub 2008 Aug 29. Biophys J. 2008. PMID: 18757561 Free PMC article.
References
-
- Chudakov DM, Lukyanov S, Lukyanov KA. Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol. 2005;23:605–613. - PubMed
-
- Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol. 2002;3:906–918. - PubMed
-
- Schultz C, Schleifenbaum A, Goedhart J, Gadella TW., Jr Multiparameter imaging for the analysis of intracellular signaling. Chembiochem. 2005;6:1323–1330. - PubMed
-
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. - PubMed
-
- Miyawaki A. Visualization of the spatial and temporal dynamics of intracellular signaling. Dev Cell. 2003;4:295–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

