Tumor suppressor CYLD acts as a negative regulator for non-typeable Haemophilus influenza-induced inflammation in the middle ear and lung of mice
- PMID: 17925880
- PMCID: PMC2001183
- DOI: 10.1371/journal.pone.0001032
Tumor suppressor CYLD acts as a negative regulator for non-typeable Haemophilus influenza-induced inflammation in the middle ear and lung of mice
Abstract
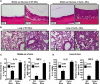
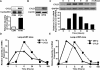
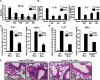
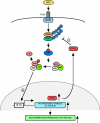
Non-typeable Haemophilus influenza (NTHi) is an important human pathogen causing respiratory tract infections in both adults and children. NTHi infections are characterized by inflammation, which is mainly mediated by nuclear transcription factor kappaB (NF-kappaB)-dependent production of inflammatory mediators. The deubiquitinating enzyme cylindromatosis (CYLD), loss of which was originally reported to cause a benign human syndrome called cylindromatosis, has been identified as a key negative regulator for NF-kappaB in vitro. However, little is known about the role of CYLD in bacteria-induced inflammation in vivo. Here, we provided direct evidence for the negative role of CYLD in NTHi-induced inflammation of the mice in vivo. Our data demonstrated that CYLD is induced by NTHi in the middle ear and lung of mice. NTHi-induced CYLD, in turn, negatively regulates NTHi-induced NF-kappaB activation through deubiquitinating TRAF6 and 7 and down-regulates inflammation. Our data thus indicate that CYLD acts as a negative regulator for NF-kappaB-dependent inflammation in vivo, hence protecting the host against detrimental inflammatory response to NTHi infection.
Conflict of interest statement
Figures


Similar articles
-
CYLD negatively regulates nontypeable Haemophilus influenzae-induced IL-8 expression via phosphatase MKP-1-dependent inhibition of ERK.PLoS One. 2014 Nov 12;9(11):e112516. doi: 10.1371/journal.pone.0112516. eCollection 2014. PLoS One. 2014. PMID: 25389768 Free PMC article.
-
NF-kappaB is essential for induction of CYLD, the negative regulator of NF-kappaB: evidence for a novel inducible autoregulatory feedback pathway.J Biol Chem. 2004 Aug 27;279(35):36171-4. doi: 10.1074/jbc.M406638200. Epub 2004 Jun 28. J Biol Chem. 2004. PMID: 15226292
-
Synergistic and feedback signaling mechanisms in the regulation of inflammation in respiratory infections.Cell Mol Immunol. 2012 Mar;9(2):131-5. doi: 10.1038/cmi.2011.65. Epub 2012 Feb 6. Cell Mol Immunol. 2012. PMID: 22307042 Free PMC article. Review.
-
Activation of epidermal growth factor receptor is required for NTHi-induced NF-κB-dependent inflammation.PLoS One. 2011;6(11):e28216. doi: 10.1371/journal.pone.0028216. Epub 2011 Nov 23. PLoS One. 2011. PMID: 22132240 Free PMC article.
-
CYLD: a multifunctional deubiquitinase.Fly (Austin). 2007 Nov-Dec;1(6):330-2. doi: 10.4161/fly.5399. Epub 2007 Nov 10. Fly (Austin). 2007. PMID: 18820455 Review.
Cited by
-
PKCθ synergizes with TLR-dependent TRAF6 signaling pathway to upregulate MUC5AC mucin via CARMA1.PLoS One. 2012;7(1):e31049. doi: 10.1371/journal.pone.0031049. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22303480 Free PMC article.
-
The Met1-linked ubiquitin machinery in inflammation and infection.Cell Death Differ. 2021 Feb;28(2):557-569. doi: 10.1038/s41418-020-00702-x. Epub 2021 Jan 20. Cell Death Differ. 2021. PMID: 33473179 Free PMC article. Review.
-
The Deubiquitinating Enzyme Cylindromatosis Dampens CD8+ T Cell Responses and Is a Critical Factor for Experimental Cerebral Malaria and Blood-Brain Barrier Damage.Front Immunol. 2017 Feb 1;8:27. doi: 10.3389/fimmu.2017.00027. eCollection 2017. Front Immunol. 2017. PMID: 28203236 Free PMC article.
-
Resveratrol suppresses NTHi-induced inflammation via up-regulation of the negative regulator MyD88 short.Sci Rep. 2016 Sep 28;6:34445. doi: 10.1038/srep34445. Sci Rep. 2016. PMID: 27677845 Free PMC article.
-
Differential regulation of Streptococcus pneumoniae-induced human MUC5AC mucin expression through distinct MAPK pathways.Am J Transl Res. 2009 May 8;1(3):300-11. Am J Transl Res. 2009. PMID: 19956440 Free PMC article.
References
-
- Rao VK, Krasan GP, Hendrixson DR, Dawid S, St Geme JW., 3rd Molecular determinants of the pathogenesis of disease due to non-typable Haemophilus influenzae. FEMS Microbiol Rev. 1999;23:99–129. - PubMed
-
- Sethi S, Murphy TF. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1969–1970. - PubMed
-
- Murphy TF. Haemophilus influenzae in chronic bronchitis. Semin Respir Infect. 2000;15:41–51. - PubMed
-
- Murphy TF. The role of bacteria in airway inflammation in exacerbations of chronic obstructive pulmonary disease. Curr Opin Infect Dis. 2006;19:225–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

