QM/MM metadynamics study of the direct decarboxylation mechanism for orotidine-5'-monophosphate decarboxylase using two different QM regions: acceleration too small to explain rate of enzyme catalysis
- PMID: 17927240
- PMCID: PMC3163499
- DOI: 10.1021/jp074858n
QM/MM metadynamics study of the direct decarboxylation mechanism for orotidine-5'-monophosphate decarboxylase using two different QM regions: acceleration too small to explain rate of enzyme catalysis
Abstract
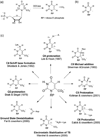
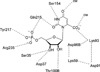
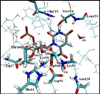
Despite decades of study, the mechanism by which orotidine-5'-monophosphate decarboxylase (ODCase) catalyzes the decarboxylation of orotidine monophosphate remains unresolved. A computational investigation of the direct decarboxylation mechanism has been performed using mixed quantum mechanical/molecular mechanical (QM/MM) dynamics simulations. The study was performed with the program CP2K that integrates classical dynamics and ab initio dynamics based on the Born-Oppenheimer approach. Two different QM regions were explored. The free energy barriers for direct decarboxylation of orotidine-5'-monophosphate (OMP) in solution and in the enzyme (using the larger QM region) were determined with the metadynamics method to be 40 and 33 kcal/mol, respectively. The calculated change in activation free energy (DeltaDeltaG++) on going from solution to the enzyme is therefore -7 kcal/mol, far less than the experimental change of -23 kcal/ mol (for k(cat.)/k(uncat.): Radzicka, A.; Wolfenden, R., Science 1995, 267, 90-92). These results do not support the direct decarboxylation mechanism that has been proposed for the enzyme. However, in the context of QM/MM calculations, it was found that the size of the QM region has a dramatic effect on the calculated reaction barrier.
Figures





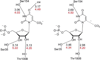

References
-
- Radzicka A, Wolfenden R. Science. 1995;267:90–92. - PubMed
-
- Steinberger R, Westheimer FH. J. Am. Chem. Soc. 1951;73:429–435.
-
- Warren S, Zerner B, Westheimer FH. Biochemistry. 1966;5:817–823. - PubMed
-
- Cleland WW. Acc. Chem. Res. 1999;32:862–868.
-
- Shostack JA, Jones ME. Biochemistry. 1992;31:12162–12168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

