Differential responsiveness to immunoablative therapy in refractory rheumatoid arthritis is associated with level and avidity of anti-cyclic citrullinated protein autoantibodies: a case study
- PMID: 17927821
- PMCID: PMC2212565
- DOI: 10.1186/ar2309
Differential responsiveness to immunoablative therapy in refractory rheumatoid arthritis is associated with level and avidity of anti-cyclic citrullinated protein autoantibodies: a case study
Abstract
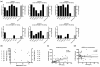
In order to identify pathogenic correlates of refractory rheumatoid arthritis (RA), antibodies against anti-cyclic citrullinated protein (ACPAs) were investigated in RA patients in whom the dysregulated immune system had been ablated by high-dose chemotherapy (HDC) and autologous haematopoietic stem cell transplantation (HSCT). Six patients with refractory RA were extensively characterized in terms of levels of total immunoglobulins, RA-specific autoantibodies (ACPAs and rheumatoid factor) and antibodies against rubella, tetanus toxoid (TT) and phosphorylcholine before and after HDC plus HSCT. Additionally, the avidity of ACPAs was measured before and after treatment and compared with the avidity of TT antibodies following repeated immunizations. Synovial biopsies were obtained by arthroscopy before HDC plus HSCT, and analyzed by immunohistochemistry. In the three patients with clinically long-lasting responses to HDC plus HSCT (median 423 days), significant reductions in ACPA-IgG levels after therapy were observed (median level dropped from 215 to 34 arbitrary units/ml; P = 0.05). In contrast, stable ACPA-IgG levels were observed in three patients who relapsed shortly after HDC plus HSCT (median of 67 days). Clinical responders had ACPA-IgG of lower avidity (r = 0.75; P = 0.08) and higher degree of inflammation histologically (r = 0.73; P = 0.09). Relapse (after 38 to 530 days) in all patients was preceded by rising levels of low avidity ACPA-IgG (after 30 to 388 days), in contrast to the stable titres of high avidity TT antibodies. In conclusion, humoral autoimmune responses were differentially modulated by immunoablative therapy in patients with synovial inflammation and low avidity ACPA-IgG autoantibodies as compared with patients with high levels of high avidity ACPA-IgG. The distinct clinical disease course after immunoablative therapy based on levels and avidity of ACPA-IgG indicates that refractory RA is not a single disease entity.
Figures

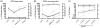
Similar articles
-
Anti-citrullinated protein antibodies have a low avidity compared with antibodies against recall antigens.Ann Rheum Dis. 2011 Feb;70(2):373-9. doi: 10.1136/ard.2010.135509. Epub 2010 Nov 10. Ann Rheum Dis. 2011. PMID: 21068094
-
Evidence for a functional role of IgE anticitrullinated protein antibodies in rheumatoid arthritis.Proc Natl Acad Sci U S A. 2010 Feb 9;107(6):2586-91. doi: 10.1073/pnas.0913054107. Epub 2010 Jan 25. Proc Natl Acad Sci U S A. 2010. Retraction in: Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):20345. doi: 10.1073/pnas.1320459110. PMID: 20133791 Free PMC article. Retracted.
-
Anti-citrullinated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients.Arthritis Rheum. 2013 Jun;65(6):1439-47. doi: 10.1002/art.37911. Arthritis Rheum. 2013. PMID: 23450693
-
Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis.Autoimmun Rev. 2010 Jan;9(3):140-3. doi: 10.1016/j.autrev.2009.04.006. Epub 2009 May 7. Autoimmun Rev. 2010. PMID: 19427413 Review.
-
Autoantibodies in rheumatoid arthritis: rheumatoid factors and anticitrullinated protein antibodies.QJM. 2010 Mar;103(3):139-46. doi: 10.1093/qjmed/hcp165. Epub 2009 Nov 19. QJM. 2010. PMID: 19926660 Free PMC article. Review.
Cited by
-
EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs.Ann Rheum Dis. 2010 Jun;69(6):964-75. doi: 10.1136/ard.2009.126532. Epub 2010 May 5. Ann Rheum Dis. 2010. PMID: 20444750 Free PMC article. Review.
-
Bortezomib: a proteasome inhibitor for the treatment of autoimmune diseases.Inflammopharmacology. 2021 Oct;29(5):1291-1306. doi: 10.1007/s10787-021-00863-2. Epub 2021 Aug 23. Inflammopharmacology. 2021. PMID: 34424482 Review.
-
Autoantibodies to posttranslational modifications in rheumatoid arthritis.Mediators Inflamm. 2014;2014:492873. doi: 10.1155/2014/492873. Epub 2014 Mar 23. Mediators Inflamm. 2014. PMID: 24782594 Free PMC article. Review.
-
A reverse translational study on the effect of rituximab, rituximab plus belimumab, or bortezomib on the humoral autoimmune response in SLE.Rheumatology (Oxford). 2020 Oct 1;59(10):2734-2745. doi: 10.1093/rheumatology/kez623. Rheumatology (Oxford). 2020. PMID: 31951278 Free PMC article.
-
Stem cell therapy: resetting autoimmunity or postponing the inevitable?Ther Adv Musculoskelet Dis. 2011 Jun;3(3):127-31. doi: 10.1177/1759720X11402117. Ther Adv Musculoskelet Dis. 2011. PMID: 22870472 Free PMC article. No abstract available.
References
-
- Aletaha D, Smolen JS. Effectiveness profiles and dose dependent retention of traditional disease modifying antirheumatic drugs for rheumatoid arthritis. An observational study. J Rheumatol. 2002;29:1631–1638. - PubMed
-
- van der Kooij SM, Vries-Bouwstra JK, Goekoop-Ruiterman YP, van Zeben D, Kerstens PJ, Gerards AH, van Groenendael JH, Hazes JM, Breedveld FC, Allaart CF, Dijkmans BA. Limited efficacy of conventional DMARDs after initial methotrexate failure in patients with recent onset rheumatoid arthritis treated according to the Disease Activity Score. Ann Rheum Dis. 2007;66:1356–1362. doi: 10.1136/ard.2006.066662. - DOI - PMC - PubMed
-
- Burt RK, Marmont A, Oyama Y, Slavin S, Arnold R, Hiepe F, Fassas A, Snowden J, Schuening F, Myint H, et al. Randomized controlled trials of autologous hematopoietic stem cell transplantation for autoimmune diseases: the evolution from myeloablative to lymphoablative transplant regimens. Arthritis Rheum. 2006;54:3750–3760. doi: 10.1002/art.22256. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

