Structure and mechanics of integrin-based cell adhesion
- PMID: 17928215
- PMCID: PMC2443699
- DOI: 10.1016/j.ceb.2007.08.002
Structure and mechanics of integrin-based cell adhesion
Abstract
Integrins are alpha/beta heterodimeric adhesion glycoprotein receptors that regulate a wide variety of dynamic cellular processes such as cell migration, phagocytosis, and growth and development. X-ray crystallography of the integrin ectodomain revealed its modular architecture and defined its metal-dependent interaction with extracellular ligands. This interaction is regulated from inside the cell (inside-out activation), through the short cytoplasmic alpha and beta integrin tails, which also mediate biochemical and mechanical signals transmitted to the cytoskeleton by the ligand-occupied integrins, effecting major changes in cell shape, behavior, and fate. Recent advances in the structural elucidation of integrins and integrin-binding cytoskeleton proteins are the subjects of this review.
Figures


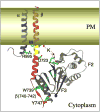
References
-
- Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. - PubMed
-
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. - PubMed
-
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–4613. - PubMed
-
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. - PubMed
-
- Michishita M, Videm V, Arnaout MA. A novel divalent cation-binding site in the A domain of the beta 2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell. 1993;72:857–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

