Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis
- PMID: 17928269
- PMCID: PMC3702386
- DOI: 10.1016/j.tube.2007.08.009
Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis
Abstract
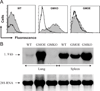
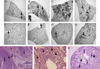
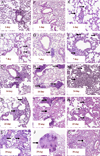
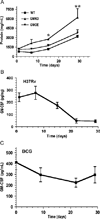
The mechanisms by which GM-CSF mediates bacterial clearance and inflammation during mycobacterial infection are poorly understood. The objective of this work was to determine how GM-CSF alters pulmonary mycobacterial infection in vivo. Differences in GM-CSF levels in the lungs of normal mice (GM(+/+)), transgenic GM-CSF-deficient (GM-CSF(-/-)), and transgenic mice with high GM-CSF expression only in lung epithelial cells (SP-C-GM-CSF(+/+)/GM(-/-)) did not affect pulmonary infection rates caused by either the attenuated Mycobacterium bovis BCG or the virulent Mycobacterium tuberculosis H37Rv. However, in contrast to findings with BCG, all GM-CSF(-/-) and SP-C-GM-CSF(+/+)/GM(-/-) mice succumbed prematurely to virulent H37Rv. Granuloma formation was impaired in both GM-CSF(-/-) and SP-C-GM-CSF(+/+)/GM(-/-) mice regardless of mycobacterial virulence. However, H37Rv-infected GM-CSF(-/-) mice suffered broncho-alveolar destruction, edema, and necrosis while only short-lived granulomas were observed in SP-C-GM-CSF(+/+)/GM(-/-) mice. Bone marrow-derived macrophages, but not dendritic cells of SP-C-GM-CSF(+/+)/GM(-/-) mice, were hypo-responsive to mycobacterial infection. Surfactant protein levels were differentially influenced by BCG and H37Rv. We conclude that GM-CSF has an essential protective role first in preserving alveolar structure and second in regulating macrophages and dendritic cells to facilitate containment of virulent mycobacteria in pulmonary granulomas. However, precise regulation of lung GM-CSF is vital to effective control of M. tuberculosis.
Figures



Similar articles
-
Role of Granulocyte-Macrophage Colony-Stimulating Factor Production by T Cells during Mycobacterium tuberculosis Infection.mBio. 2017 Oct 24;8(5):e01514-17. doi: 10.1128/mBio.01514-17. mBio. 2017. PMID: 29066547 Free PMC article.
-
Keratinocyte growth factor administration attenuates murine pulmonary mycobacterium tuberculosis infection through granulocyte-macrophage colony-stimulating factor (GM-CSF)-dependent macrophage activation and phagolysosome fusion.J Biol Chem. 2015 Mar 13;290(11):7151-9. doi: 10.1074/jbc.M114.591891. Epub 2015 Jan 20. J Biol Chem. 2015. PMID: 25605711 Free PMC article.
-
Pulmonary surfactant and tuberculosis.Tuberculosis (Edinb). 2009 Dec;89 Suppl 1:S10-4. doi: 10.1016/S1472-9792(09)70005-8. Tuberculosis (Edinb). 2009. PMID: 20006297
-
Granulocyte-macrophage colony-stimulating factor and pulmonary surfactant homeostasis.Proc Assoc Am Physicians. 1998 Jul-Aug;110(4):321-32. Proc Assoc Am Physicians. 1998. PMID: 9686680 Review.
-
Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense.Annu Rev Physiol. 2002;64:775-802. doi: 10.1146/annurev.physiol.64.090601.113847. Annu Rev Physiol. 2002. PMID: 11826288 Review.
Cited by
-
Analysis of alpha-1-antitrypsin (AAT)-regulated, glucocorticoid receptor-dependent genes in macrophages reveals a novel host defense function of AAT.Physiol Rep. 2024 Jul;12(14):e16124. doi: 10.14814/phy2.16124. Physiol Rep. 2024. PMID: 39016119 Free PMC article.
-
Role of Granulocyte-Macrophage Colony-Stimulating Factor Production by T Cells during Mycobacterium tuberculosis Infection.mBio. 2017 Oct 24;8(5):e01514-17. doi: 10.1128/mBio.01514-17. mBio. 2017. PMID: 29066547 Free PMC article.
-
Genetic variation in CSF2 (5q31.1) is associated with longitudinal susceptibility to pediatric malaria, severe malarial anemia, and all-cause mortality in a high-burden malaria and HIV region of Kenya.Trop Med Health. 2022 Jun 25;50(1):41. doi: 10.1186/s41182-022-00432-5. Trop Med Health. 2022. PMID: 35752805 Free PMC article.
-
Human Macrophages Exhibit GM-CSF Dependent Restriction of Mycobacterium tuberculosis Infection via Regulating Their Self-Survival, Differentiation and Metabolism.Front Immunol. 2022 May 12;13:859116. doi: 10.3389/fimmu.2022.859116. eCollection 2022. Front Immunol. 2022. PMID: 35634283 Free PMC article.
-
GM-CSF Dependent Differential Control of Mycobacterium tuberculosis Infection in Human and Mouse Macrophages: Is Macrophage Source of GM-CSF Critical to Tuberculosis Immunity?Front Immunol. 2020 Jul 23;11:1599. doi: 10.3389/fimmu.2020.01599. eCollection 2020. Front Immunol. 2020. PMID: 32793233 Free PMC article.
References
-
- Maher D, Raviglione M. Global epidemiology of tuberculosis. Clin Chest Med. 2005;26:167–182. v. - PubMed
-
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. - PubMed
-
- North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. - PubMed
-
- Fleetwood AJ, Cook AD, Hamilton JA. Functions of granulocy-te-macrophage colony-stimulating factor. Crit Rev Immunol. 2005;25:405–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

