Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells
- PMID: 17928334
- PMCID: PMC2224360
- DOI: 10.1128/JVI.01794-07
Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells
Abstract
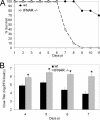
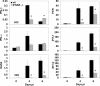
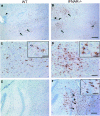
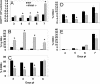
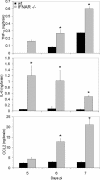
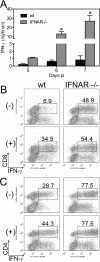
Neurotropic coronavirus infection induces expression of both beta interferon (IFN-beta) RNA and protein in the infected rodent central nervous system (CNS). However, the relative contributions of type I IFN (IFN-I) to direct, cell-type-specific virus control or CD8 T-cell-mediated effectors in the CNS are unclear. IFN-I receptor-deficient (IFNAR(-/-)) mice infected with a sublethal and demyelinating neurotropic virus variant and those infected with a nonpathogenic neurotropic virus variant both succumbed to infection within 9 days. Compared to wild-type (wt) mice, replication was prominently increased in all glial cell types and spread to neurons, demonstrating expanded cell tropism. Furthermore, increased pathogenesis was associated with significantly enhanced accumulation of neutrophils, tumor necrosis factor alpha, interleukin-6, chemokine (C-C motif) ligand 2, and IFN-gamma within the CNS. The absence of IFN-I signaling did not impair induction or recruitment of virus-specific CD8 T cells, the primary adaptive mediators of virus clearance in wt mice. Despite similar IFN-gamma-mediated major histocompatibility complex class II upregulation on microglia in infected IFNAR(-/-) mice, class I expression was reduced compared to that on microglia in wt mice, suggesting a synergistic role of IFN-I and IFN-gamma in optimizing class I antigen presentation. These data demonstrate a critical direct antiviral role of IFN-I in controlling virus dissemination within the CNS, even in the presence of potent cellular immune responses. By limiting early viral replication and tropism, IFN-I controls the balance of viral replication and immune control in favor of CD8 T-cell-mediated protective functions.
Figures


References
-
- Bahl, K., S. K. Kim, C. Calcagno, D. Ghersi, R. Puzone, F. Celada, L. K. Selin, and R. M. Welsh. 2006. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J. Immunol. 1764284-4295. - PubMed
-
- Bailey, S. L., P. A. Carpentier, E. J. McMahon, W. S. Begolka, and S. D. Miller. 2006. Innate and adaptive immune responses of the central nervous system. Crit. Rev. Immunol. 26149-188. - PubMed
-
- Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 1633379-3387. - PubMed
-
- Bergmann, C. C., B. Parra, D. R. Hinton, R. Chandran, M. Morrison, and S. A. Stohlman. 2003. Perforin-mediated effector function within the central nervous system requires IFN-gamma-mediated MHC up-regulation. J. Immunol. 1703204-3213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

