Nef-mediated enhancement of virion infectivity and stimulation of viral replication are fundamental properties of primate lentiviruses
- PMID: 17928336
- PMCID: PMC2168858
- DOI: 10.1128/JVI.00904-07
Nef-mediated enhancement of virion infectivity and stimulation of viral replication are fundamental properties of primate lentiviruses
Abstract
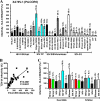
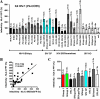
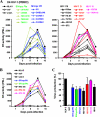
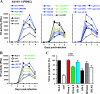
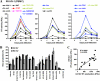
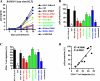
Nef is a multifunctional accessory protein of primate lentiviruses. Recently, it has been shown that the ability of Nef to downmodulate CD4, CD28, and class I major histocompatibility complex is highly conserved between most or all primate lentiviruses, whereas Nef-mediated downregulation of T-cell receptor-CD3 was lost in the lineage that gave rise to human immunodeficiency virus type 1 (HIV-1). Whether or not other Nef activities are preserved between different groups of primate lentiviruses remained to be determined. Here, we show that nef genes from a large variety of HIVs and simian immunodeficiency viruses (SIVs) enhance virion infectivity and stimulate viral replication in human cells and/or in ex vivo infected human lymphoid tissue (HLT). Notably, nef alleles from unpassaged SIVcpz and SIVsmm enhanced viral infectivity, replication, and cytopathicity in cell culture and in ex vivo infected HLT as efficiently as those from HIV-1 and HIV-2, their human counterparts. Furthermore, nef genes from several highly divergent SIVs that have not been found in humans were also highly active in human cells and/or tissues. Thus, most primate lentiviral Nefs enhance virion infectivity and stimulate viral replication. Moreover, our data show that SIVcpz and SIVsmm Nefs do not require adaptive changes to perform these functions in human cells or tissues and support the idea that nef alleles from other primate lentiviruses would also be capable of promoting efficient virus spread in humans.
Figures

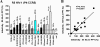
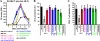
Similar articles
-
Loss of Nef-mediated CD3 down-regulation in the HIV-1 lineage increases viral infectivity and spread.Proc Natl Acad Sci U S A. 2020 Mar 31;117(13):7382-7391. doi: 10.1073/pnas.1921135117. Epub 2020 Mar 16. Proc Natl Acad Sci U S A. 2020. PMID: 32179688 Free PMC article.
-
Conservation of Nef function across highly diverse lineages of SIVsmm.Retrovirology. 2009 Apr 9;6:36. doi: 10.1186/1742-4690-6-36. Retrovirology. 2009. PMID: 19358735 Free PMC article.
-
SIVcol Nef counteracts SERINC5 by promoting its proteasomal degradation but does not efficiently enhance HIV-1 replication in human CD4+ T cells and lymphoid tissue.PLoS Pathog. 2018 Aug 20;14(8):e1007269. doi: 10.1371/journal.ppat.1007269. eCollection 2018 Aug. PLoS Pathog. 2018. PMID: 30125328 Free PMC article.
-
Role of HIV-1 Nef protein for virus replication in vitro.Microbes Infect. 2010 Jan;12(1):65-70. doi: 10.1016/j.micinf.2009.09.009. Epub 2009 Sep 19. Microbes Infect. 2010. PMID: 19770068 Review.
-
Biology of the HIV Nef protein.Indian J Med Res. 2005 Apr;121(4):315-32. Indian J Med Res. 2005. PMID: 15817946 Review.
Cited by
-
HIV Vpu Interferes with NF-κB Activity but Not with Interferon Regulatory Factor 3.J Virol. 2015 Oct;89(19):9781-90. doi: 10.1128/JVI.01596-15. Epub 2015 Jul 15. J Virol. 2015. PMID: 26178989 Free PMC article.
-
AP-2 Adaptor Complex-Dependent Enhancement of HIV-1 Replication by Nef in the Absence of the Nef/AP-2 Targets SERINC5 and CD4.mBio. 2023 Feb 28;14(1):e0338222. doi: 10.1128/mbio.03382-22. Epub 2023 Jan 9. mBio. 2023. PMID: 36622146 Free PMC article.
-
Nef enhances HIV-1 replication and infectivity independently of SERINC5 in CEM T cells.Virology. 2023 Jan;578:154-162. doi: 10.1016/j.virol.2022.12.008. Epub 2022 Dec 23. Virology. 2023. PMID: 36577173 Free PMC article.
-
Structural Basis for Tetherin Antagonism as a Barrier to Zoonotic Lentiviral Transmission.Cell Host Microbe. 2019 Sep 11;26(3):359-368.e8. doi: 10.1016/j.chom.2019.08.002. Epub 2019 Aug 22. Cell Host Microbe. 2019. PMID: 31447307 Free PMC article.
-
HIV-1 Nef-mediated downregulation of CD155 results in viral restriction by KIR2DL5+ NK cells.PLoS Pathog. 2022 Jun 24;18(6):e1010572. doi: 10.1371/journal.ppat.1010572. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35749424 Free PMC article.
References
-
- Anderson, J. L., and T. J. Hope. 2004. HIV accessory proteins and surviving the host cell. Curr. HIV/AIDS Rep. 1:47-53. - PubMed
-
- Ayouba, A., S. Souquieres, B. Njinku, P. M. Martin, M. C. Muller-Trutwin, P. Roques, F. Barre-Sinoussi, P. Mauclere, F. Simon, and E. Nerrienet. 2000. HIV-1 group N among HIV-1-seropositive individuals in Cameroon. AIDS 14:2623-2625. - PubMed
-
- Bailes, E., F. Gao, F. Bibollet-Ruche, V. Courgnaud, M. Peeters, P. A. Marx, B. H. Hahn, and P. M. Sharp. 2003. Hybrid origin of SIV in chimpanzees. Science 300:1713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

